Abstract
YVO4:Er3+ and YVO4:Er3+, Yb3+ nanomaterials were prepared via combustion synthesis using urea as fuel and metal nitrates as precursor. The morphology and the structure of the prepared samples were characterized by x-ray diffraction, scanning electron microscopy and transmission electron microscopy. The average size of the prepared materials ranged from 20 to 30 nm in diameter. The effects of Er3+ and Yb3+ doping concentrations on structure and optical properties have been investigated. Optical properties of YVO4:Er3+ and YVO4:Er3+, Yb3+ nanoparticles were measured by photoluminescent excitation and emission spectroscopies. For the YVO4:Er3+ and YVO4:Er3+, Yb3+ samples, two strong green emissions centered at 524 and 552 nm are found, corresponding to the 2H11/2—4I15/2 and 4S11/2—4I15/2 transitions of Er3+ ions, respectively. Upconversion emission in the green region of the YVO4:Er3+ nanoparticles under 980 nm excitation was also investigated. Strong emission from these materials is promising for luminescent biolabeling applications.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years studies on synthesis, optical properties and applications of rare-earth doped nanomaterials have been attracting much interest [1–6]. In particular, for many novel applications such as biological labels or security labels, properties of phosphors need to be optimized at the nanoscale [5–8]. Among these luminescent materials, upconversion luminescent materials can convert near-infrared radiation into visible radiation by emitting a higher-energy photon after absorbing multiple lower-energy photons. It was reported that efficient upconversion luminescent materials with grain size small enough could be used as new luminescent markers for the detection of biomolecules. For biological applications, upconversion luminescent nanomaterials exhibit many advantages over conventional organic dye markers and quantum dots, which are excited in the UV or blue spectral region, such as high chemical stability, low toxicity, less harmful to cells and living organisms, high light penetration depth in tissues, weak autofluorescence from cells or tissues, low background light and high sensitivity for detection [5, 8, 9].
In addition, the availability of low-cost near-infrared laser diodes (980 nm) has also stimulated research and applications in the area of upconversion luminescent materials. Materials doped with Er 3+ ions and/or codoped with Yb 3+ ions have been widely studied as Er 3+ ions possess a favorable metastable energy level with longer lifetime excited states, and Yb 3+ ions have a large absorption cross-section around 980 nm and can efficiently transfer the excitation energy to Er 3+ ions [9].
Eu 3+-activated yttrium orthovanadate (YVO 4), a well-known rare-earth (RE) doped inorganic luminescent material, has been used as an important commercial red phosphor since 1964 owing to its high luminescence efficiency on electron-beam excitation. Recently, considerable efforts have been devoted to developing different synthesis methods for the preparation of luminescent colloidal YVO 4:RE 3+ (RE=Er, Sm, Yb, etc) nanoparticles [4, 10, 11]. For example, PL and upconversion luminescence of YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ nanomaterials have been investigated [10, 11]. Only a few studies focusing on the synthesis of YVO 4:RE 3+ via a combustion method have been reported [12]. However, to our knowledge, no report on the synthesis and properties of nano-sized YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ via a combustion method is available yet.
In this paper we present the synthesis and optical properties of YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ nanomaterials synthesized via a combustion method.
2. Experimental
YVO 4:Er 3+ nanomaterials were synthesized by a combustion method from Y(NO 3)3, Er(NO 3)3, Yb(NO 3)3, NH 4 VO 3 and urea (H 2 NCONH 2). In this study, yttrium oxide (99.99%), erbium oxide (99.99%), ytterbium oxide (99.99%), NH 4 VO 3 (99.8%), nitric acid and urea (99%) were used as the starting raw materials. Y(NO 3)3, Er(NO 3)3 and Yb(NO 3)3 stock solutions were prepared by dissolving Y 2 O 3, Er 2 O 3 and Yb 2 O 3 in nitric acid and diluting with deionized water. Aqueous solutions of Y(NO 3)3 and RE(NO 3)3 were mixed to appropriate molar ratios in a glass beaker and then a suitable amount of urea was added. The mixture was dissolved by magnetic stirring until a uniform solution was achieved. The solution was then concentrated by heating at 80 °C until any excess free water evaporated. Finally, the solid residue was preheated at 500 °C in a furnace for 1 h and then calcined at temperatures in the range of 550–900 °C for 1 h.
Micro-sized YVO 4:Er 3+ materials were also synthesized by conventional solid state reaction at 1100 °C for 3 h to be used as references. Stoichiometric amounts of Y 2 O 3 (99.99%), Er 2 O 3 (99.99%) and NH 4 VO 3 (99.8%) were mixed and finely grinded in an agate mortar. This mixture was prefired for 3 h at 900 °C, then regrinded and calcined at 1100 °C for 3 h in air.
The morphology and structure of the prepared samples were characterized by x-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Photoluminescent (PL) and photoluminescent excitation (PLE) spectra were obtained at room temperature using a Spex Fluorolog-3 spectrophotometer with 450 W Xe light sources. Emission and upconversion emission spectra of YVO 4:Er 3+ nanoparticles were also obtained by using an iHR 550 system under 266 and 980 nm excitation (by a diode laser).
3. Results and discussion
The XRD patterns of the resulting products YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+, were indexed to tetragonal YVO 4 phase without any other crystalline phase being observed (JCPDS card 17-341). Figure 1 displays the XRD patterns of YVO 4:Er 3+ samples annealed at 550–900 °C for 1 h. The formation of YVO 4 phase was completely obtained at temperature as low as 550 °C and for calcination time as short as 1 h by using a combustion method. This is quite an impressive advantage of the preparative route using a combustion method in comparison with a solid-state reaction method. For the latter case, only a small amount of YVO 4 phase could be obtained after calcination at 900 °C for 3 h and it is necessary to carry out further heat treatment process at 1100 °C for 3 h to obtain the single phase of the YVO 4 host material (results are not shown here).
Figure 1 XRD patterns of YVO 4:Er 3+ (2 at.%) calcined for 1 h at (a) 550 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C and (e) 900 °C. Vertical lines belong to JCPDS card No. 17-0341 of YVO 4.
From figure 1, the broadening of diffraction peaks clearly indicates that small nanocrystals are present in the samples. In addition, the diffraction peak widths of samples calcined at different temperatures ranging from 550 to 900 °C were almost the same. This revealed that the crystal size of the prepared samples only slightly depends on calcination temperature in the range from 550 to 900 °C. The crystal size of the sample can be estimated by Scherrer's formula: , where D is the average diameter of the grains, λ is the wavelength of the x-ray (λ=0.1541 nm), β and θ are the full width at half maximum (FWHM) of the XRD lines and the Bragg angle, respectively. The average particle size was estimated to be 19 and 20 nm for the samples heated at 550 and 900 °C, respectively.
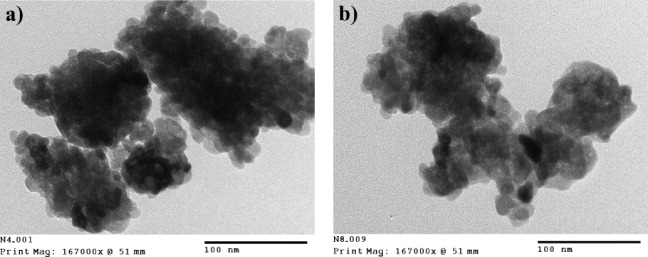
Similar to the XRD results, SEM images show that no significant effect of calcination temperature on the crystal size of YVO 4:Er 3+ nanomaterials calcined at 550–900 °C for 1 h was found with a narrow particle size distribution from 20 to 30 nm. Figure 2 presents TEM images of YVO 4:Er 3+ samples calcined at 550 and 900 °C for 1 h. One can see from figure 2 that the average particle size of YVO 4:Er 3+ nanoparticles can range from 20 to 30 nm. XRD patterns of YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ calcined at 700 °C for 1 h do not show any significant difference between samples doped with low doping concentration (0.5 at.% Er) and with high concentration (10 at.% Er or 2 at.% Er and 10 at.%Yb).
Figure 2 TEM images of YVO 4:Er 3+ (2 at.%) calcined at (a) 550 °C and (b) 900 °C.
To investigate the optical properties of the prepared materials, their PLE and PL spectra were measured. Figure 3 shows the PLE spectrum of the YVO 4:Er 3+ sample prepared by a combustion method. Six excitation peaks at 367, 379.5, 408, 452.5, 490 and 522 nm in the PLE spectrum are attributed to transitions from ground state to excited states of Er 3+ ions: 4 I 15/2 →2 K 15/2, 4 I 15/2 →4 G 11/2, 4 I 15/2 →2 H 9/2, 4 I 15/2 →4 F 5/2, 3/2, 4 I 15/2 →4 F 7/2 and 4 I 15/2 →2 H 11/2, respectively [5, 13, 14]. In addition, the PLE spectrum consists of a broad intense band at around 310 nm which is contributed by the VO 4 3- group in the host matrix [9, 15, 16]. This observation indicates that the emission of Er 3+ ions occurs via energy transfers from the excited VO 4 3- group.
Figure 3 PLE spectrum (monitored by 552 nm) of YVO 4:Er 3+ (2 at.%) calcined at 700 °C. The dotted line presents the higher magnification PLE spectrum.
PL spectra of YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ under 266 nm excitation are presented in figure 4. Upon 266 nm excitation, which is the host absorption, two strong green emissions centered around 524 and 552 nm were observed in the PL spectra of the YVO 4:Er 3+ nanoparticles. Emissions at 520–535 and 543–560 nm correspond to the 2 H 11/2—4 I 15/2 and 4 S 11/2—4 I 15/2 transitions of Er 3+ ions, respectively [2, 10, 11]. Very weak emission in the red region of 650–675 nm corresponding to the 4 F 9/2 →4 I 15/2 transitions of Er 3+ ion was also detected. The explanation is that the vanadate groups absorbed UV excitation and transferred energy to the erbium ions, and the emissions in the green and red regions could then be observed. The origin of these emissions is due to the electronic transitions of Er 3+. As a result, it is quite understandable that PL intensity under 266 nm excitation was not significantly affected by the addition of Yb 3+ (figure 4). Figure 5 shows PL spectra of YVO 4:Er 3+ nanoparticles under 266 nm excitation with Er 3+ concentration of 0.5, 1, 2, 5 and 10 at.%. The emission intensity increased with Er 3+ atomic concentration and reached a maximum value with 5 at.% Er 3+.
Figure 4 PL spectra of YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ nanoparticles calcined at 700 °C under 266 nm excitation. The inset is a luminescent photograph of the YVO 4:Er 3+ (2 at.%) sample.
Figure 5 PL spectra of YVO 4:Er 3+ nanoparticles calcined at 700 °C with different molar concentrations of Er 3+ . The inset shows the PL intensities of these samples at 524 nm.
As mentioned above, the crystal size of the prepared samples was almost unchanged for the calcination temperature range of 550–900 °C but the difference in PL intensity of the YVO 4:Er 3+ samples was clearly seen. Figure 6 shows the PL spectra and PL intensity of YVO 4:Er 3+ (2 at.%) nanoparticles versus calcination temperature. It is shown that PL intensity increases with calcination temperature.
Figure 6 The dependence of PL spectra of the YVO 4:Er 3+ (2 at.%) nanoparticles on calcination temperature.
The above emission is contributed by the VO 4 3- group in the host matrix by UV excitation where the energy of emission is lower than that of excitation. From the PLE spectrum, it is seen that Er 3+ ions can directly absorb energy at 367, 379.5, 408, 452.5, 490 and 522 nm to populate in 2 K 15/2, 4 G 11/2, 2 H 9/2, 4 F 5/2, 3/2, 4 F 7/2 and 2 H 11/2 states. After nonradiative relaxation from these states to 2 H 11/2 and 4 S 11/2 states, green emission can be observed. It is very interesting that YVO 4:Er 3+ nanoparticles can absorb lower energy (at 980 nm) than 2 H 11/2 and 4 S 11/2 states and provide a green emission, which is called the upconversion luminescent process. Figure 7 shows the upconversion luminescent spectrum of YVO 4:Er 3+ nanoparticles under 980 nm laser excitation. The sharp peaks in the green region of 520–560 nm correspond to the (2 H 11/2, 4 S 3/2) →4 I 15/2 transitions of Er 3+ [10, 11]. In particular, the intensity of these peaks emitted from nano-sized samples is higher than that from micro-sized ones. The upconversion luminescent process in Er 3+ ions of our prepared YVO 4:Er 3+ samples can be explained by using an energy diagram as shown in figure 7 [10, 11, 14, 17]. For the excited-state absorption (ESA) process, a 980 nm photon from the laser excites the Er 3+ ion from the ground-state 4 I 15/2 to the excited state 4 I 11/2. After the first-level excitation, the same laser pumps the excited atom from the 4 I 11/2 to the 4 F 7/2 level [10, 16]. For the energy transfer upconversion (ETU) process, two excited Er 3+ ions at 4 I 11/2 interact each with other, one is de-excited to 4 I 15/2, and another is excited to the 4 F 7/2 level [10, 13]. Subsequent nonradiative relaxation populates the 2 H 11/2 and 4 S 3/2 levels. Bright green emission is observed owing to the 2 H 11/2, 4 S 3/2→4 I 15/2 transitions.
Figure 7 (a) Upconversion luminescent spectra of YVO 4:Er 3+ (2 at.%) nano-sized particles prepared by the combustion method (red line) and YVO 4:Er 3+ (2 at.%) micro-sized particles prepared by solid-state reaction method (green line) under 980 nm excitation. The inset is an upconversion luminescent photograph of the YVO 4:Er 3+ nano sample. (b) Energy diagram of the upconversion luminescent process in Er 3+ ions.
4. Conclusion
YVO 4:Er 3+ and YVO 4:Er 3+, Yb 3+ nanomaterials were successfully synthesized via a combustion method at 550–900 °C for 1 h. The average size of the nanoparticles is about 20–30 nm in diameter and almost unchanged with calcination temperatures in the range of 550–900 °C. YVO 4:Er 3+ nanoparticles emit a green color corresponding to the emission transitions of Er 3+ ions under the excitation of both infrared (980 nm) and UV (266 nm) radiation. It is very interesting for further applications such as luminescent biolabeling.
Acknowledgment
The present research was supported by grants from National Foundations for Science and Technology Development (NAFOSTED) code 103.03.81.09. Part of the work was done in the National Key Laboratory for Electronic Materials and Devices, Institute of Materials Science (VAST).