Abstract
The use of carbon nanotubes (CNTs) in radio frequency identification (RFID) applications offers a very large range of possibilities to exploit the incredible properties of CNTs. However, due to their entanglement state, their size and the different interacting forces between nanotubes bundles present at nanometric scale, CNTs debundling is very hard to achieve, requiring specific equipment and chemicals. Our purpose was to reduce as small as possible CNTs bundles, in order to realize ink to print on an RFID antenna. The size of the head printer nozzles required very small particles, about a few micrometers, in order to be able to print on the sensitive position of the antenna. To reduce the size of the bundles and stabilize the solution, an ultrasonic horn with an ultrasonic bath were combined as mechanical stress for CNT dispersion, and some chemicals such as sodium dodecyl sulfate (SDS)—a surfactant, N-methyl-2-pyrrolidone (NMP)—a solvent, or chitosan were used to meet our requirements.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since their discovery in 1991, carbon nanotubes (CNTs) have attracted the interest of many scientists and industrialists due to their incredible properties and the very large range of applications where they can be used. Their mechanical, electrical and thermal properties allow using them in very specific applications.
Although it is very difficult to obtain a CNTs solution suitable for inkjet printer, many researchers are trying to obtain usable CNT inks for their applications [1–7]. At nanoscale, the different forces in CNT bundles represent a challenge to disperse CNTs in the medium. Van der Waals interactions and π-stacking cause a problem for CNT dispersion stable in time. At microscale, the entanglement state of the CNTs, the presence of impurities, the sedimentation of the particles and the environment are key parameters that need to be controlled in order to achieve suitable CNT dispersion.
Currently, many ways to disperse CNTs exist such as mechanical stress and chemical modifications [8, 9]. Our project required CNT particles smaller than a few micrometers. The first classical way is to use a mechanical stress as dispersant. The problem of this technique is that CNTs are hydrophobic materials and need some specific treatments or specific medium to be disperse. So many different chemicals were used to obtain a suitable solution.
In the first step, chitosan was used as a stabilizer for the dispersion. The interest for chitosan arises because it is a material based on chitin extracted from crustaceans. It represents a renewable material with good properties such as the sensitivity for some gases like NH3 and can be useful for our application of RFID antenna. Steric stabilization techniques were used to disperse CNT into chitosan and then obtain stable and homogenous CNT dispersion.
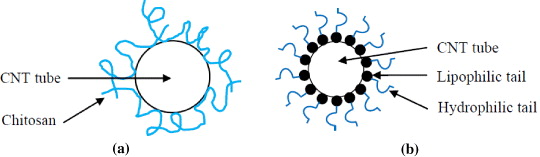
In the second step, sodium dodecyl sulfate (SDS), which shows a great capacity to disperse CNT into aqueous medium [8, 10, 11] was used as a surfactant. It is composed of hydrophilic heads, which allows good interaction in water and has lipophilic tails that interact with a CNT wall. The mechanism of interaction between SDS and a CNT tube is shown in figure 1(b).
Figure 1. (a) Steric hindrance scheme between CNT and chitosan and (b) SDS interaction with CNT wall.
Download figure:
Standard image High-resolution imageIn a final step, dispersion of CNT into N-methyl-2-pyrrolidone (NMP) was performed. NMP is a well-known solvent for its capacity to disperse CNT at low concentration.
2. Experimental
2.1. Material
CNTs were supplied by Sigma Aldrich; NMP and SDS were supplied by Merck; chitosan was supplied by Hi-Media. The devices used for this project consist of an ultrasonic horn 'Sonic Ruptor' supplied by Omni International, an ultrasonic bath 'Branson 1510', a centrifuge device 'Rotina 38' and 5 μm paper filter supplied by Advantec.
2.2. Chitosan/CNT
Our first experiment was to try to achieve CNT dispersion by using chitosan, which offers many interesting properties to disperse CNTs [12–14]. A solution composed of 0.03 g of chitosan, 20 ml of de-ionized water and 1 wt% of acetic acid was prepared in order to obtain a suitable, stable and homogenous dispersion of chitosan (figure 2(a)). In order to obtain dispersion without impurities, a first filtration using a 5 μm paper filter was realized. Then CNTs were dispersed inside chitosan solution (figure 2(b)).
Figure 2. (a) Chitosan dispersion in water and (b) CNT dispersion in chitosan.
Download figure:
Standard image High-resolution image2.3. SDS/CNT
In order to get a good solution, the concentration of CNTs was fixed as 2 mg ml−1 and an ultrasonic horn device was used (figure 3(a)). The key parameters to obtain a suitable solution are amplitude of the ultrasonic device, pulser time and duration. The process was optimized by using a 1 h cycle with 50% amplitude and 2 s on, 0.5 s off pulse.
Figure 3. (a) Ultrasonic horn device and (b) combination of ultrasonic horn and ultrasonic bath.
Download figure:
Standard image High-resolution imageThe ultrasonic horn needed to be used carefully, because it could influence the solution reaction process. Indeed, when a high rate of amplitude is used, cavitation phenomenon occurs at the contact point between the horn and the surface of the solution.
After using the ultrasonic device, a chitosan/CNT solution, which meets our requirements of viscosity between 8 and 12 cps and surface tension about 33 dynes was achieved. The solution was used for printing by an inkjet printer, but it was unsuccessful because of chitosan existence. Actually, the nozzles' size (about 21.5 μm) does not allow printing with the use of chitosan. Chitosan is composed of great macromolecular chains and needs time to re-organize itself in the flow direction, but the inkjet printing process is an 'instantaneous' jet of material. Therefore, chitosan cannot be ejected by the nozzles and this leads to sealing of the nozzles.
After the failure of printing CNTs using chitosan, chemicals were used in order to be able to disperse CNTs into water. A solution composed of de-ionized water, 1 wt% sodium dodecyl sulfate and 2 mg ml−1 of CNTs was prepared. In order to break the bundles into smaller ones and avoid the cavitation phenomena, both an ultrasonic horn and an ultrasonic bath were used at the same time for 1 h (figure 3(b)). The conditions of the ultrasonic horn device were the same as in the previous experiment with CNT/chitosan (50% amplitude and 2 s on, 0.5 s off pulse). The amplitude is limited at 50% because above this value cavitation phenomenon appears and compromises the dispersion of CNTs.
Then a centrifuge was used in order to separate the big CNT aggregates into smaller ones. The ultrasonication and the centrifugation cycles were repeated about five times, until there were no aggregates remaining at the bottom of the vials. A homogenous and stable solution in SDS was achieved and filtered. The size of the nozzles limited the particle size inside the solution to about a few micrometers. Therefore, the solution must be filtered at 5 μm by filter paper. After that, this solution was used for printing on silver substrate.
2.4. NMP/CNT
The third solution consisted of N-methyl-2-pyrrolidone (NMP), a well-known solvent for its effectiveness in dispersing CNTs [15, 16]. CNTs were dispersed into a pure NMP solution without any aqueous surfactant, and a 1 h cycle of ultrasonication was used, followed by a 5 μm filtration. A great loss of CNTs was observed during the filtration step, and a light gray solution was obtained. However, the obtained solution is not good because of it sediment after 3 days. The NMP solution was not suitable for printing, so the SDS/CNT solution became our main purpose.
3. Results and discussion
SDS/CNT solution appeared as the most suitable for our purpose. The mechanism of CNT dispersion by SDS is now well known and controlled. Sodium dodecyl sulfate is an amphiphilic surfactant composed of two parts, a hydrophilic head that interacts with water and a lypophilic tail that interacts with CNTs. Due to different interactions between the two compounds, SDS offers steric hindrance capacities, which allow us to obtain a stable and homogenous solution. Indeed, when SDS was added with the presence of CNTs, there appeared micelles formation between the wall of the CNT and water. Then, when SDS was well dispersed in water, it covered all the surface of the nanotube.
Thanks to SDS, CNT becomes stable in the water medium. However, its concentration has to be controlled because if the concentration is too high, SDS can interact with itself instead of CNT and does not interact with CNT anymore (critical micelle concentration). Furthermore, keep in mind that due to its presence on the surface of the nanotubes, SDS could cause a loss of conductivity of the CNTs. Therefore, 1 wt% concentration of SDS to disperse CNTs was chosen, which represented the best compromise between the CNTs dispersion and the risk of CNT insulation.
Then, in order to use our solution for the printing, the size of aggregates must be characterized. The nozzles' size is 21.5 μm, so particles have to be about ten times smaller than this size, about a few micrometers. Indeed, if bigger aggregates are used, this represents the risk of sealing the nozzles. However, due to this size, a great loss of CNTs during the filtration step was observed, and so a loss of CNT concentration took place inside the solution. In order to meet our printer requirement dealing with the necessary viscosity (8–12 cps) and the necessary surface tension (33 dynes), a set of chemicals were added, whose properties are well known and controlled. The set of chemicals used to fit the solution properties is composed of ethylene glycol, glycerin and 2-isopropoxyethanol.
Figure 4 shows two solutions: left is CNT solution after filtration by filter paper 5 μm and right is CNT solution after adding ethylene glycol, glycerin and 2-isopropoxyethanol in order to control viscosity and surface tension. Before printing by inkjet printer, this solution needed to be characterized by using scanning electron microscope (SEM).
Figure 4. CNT solution after filtration step (left) and CNT ink after adding a set of chemicals (right) to control viscosity and surface tension.
Download figure:
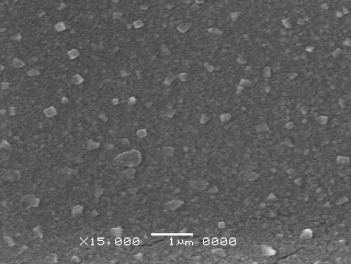
Standard image High-resolution imageBy using SEM, the CNTs' size was observed, about 3.0–4.0 μm, smaller than 5.0 μm (figure 5). This size meets requirement of the nozzle size of our inkjet printer. Therefore, this solution can be used to print on silver substrate.
Figure 5. SEM observation of SDS on CNT surface.
Download figure:
Standard image High-resolution imageSDS/CNT solution was printed on silver substrate by using an inkjet printer. Two samples were prepared, the first one is composed of silver substrate with one layer of CNT ink (figure 6(a)) and the second one is composed of three layers of CNT ink on silver substrate (figure 6(b)). An observation shows that even if it is a low-concentration CNT ink, the three-layers sample shows a difference of color on the area where CNT ink was used for the printing. Afterward, these samples were characterized by using an optical microscope and SEM in order to observe the state of dispersion and the size of the colloids particles.
Figure 6. (a) One CNT layer printing on silver substrate and (b) three CNT layers printing on silver substrate.
Download figure:
Standard image High-resolution imageFigures 7 and 8 show the dispersion of CNT ink on silver substrate by using an optical microscope and SEM. It seems pretty well dispersed and homogenous. The presence of very long nanotubes mixing with smaller aggregates of CNTs was observed. However, the size of CNT was well controlled, smaller than 5.0 μm and this ensured that the nozzle of the inkjet printer would not be sealed.
Figure 7. Optical microscope observation of three-layers CNT ink on silver substrate (a) ×10 and (b) ×100.
Download figure:
Standard image High-resolution imageFigure 8. SEM observation of one CNT layer on silver substrate.
Download figure:
Standard image High-resolution image4. Conclusion
CNT dispersion in liquid medium has been studied for chitosan solution, water and N-methyl-2-pyrrolidone (NMP) solution. The dispersion of CNTs in water was found to be one of the easier ways to obtain printable ink based on CNTs. Here a solution with low size aggregates was achieved by using the combination of two ultrasonic devices (ultrasonic horn and ultrasonic bath) and suitable surfactant (SDS).
Then a printing of our solution on silver ink pattern was tested successfully, and an observation of the state of dispersion and the size of the remaining bundles of CNT has been done. As a result, the size of about 3.0–4.0 μm for a majority of remaining aggregates was measured.
The success in the synthesis of CNT ink leads to potential applications by combining with RFID technology. Low-cost leaking gas detector or gas sensor can be made by using an inkjet printer to drop CNT ink precisely onto the sensitive position of an RFID tag. This method can be applied to create a leaking gas detector system with many advantages such as low-cost, wireless, sharp response, etc.
Acknowledgment
The authors highly appreciate the financial support of the Ministry of Sciences and Technology of Vietnam.