Abstract
In this paper we present the results of study on the magnetic and magnetocaloric properties of LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) ribbons prepared by using melt-spinning method. The results show that the glass forming ability (GFA) of the alloy is considerably influenced by the addition of Co. With increasing Co-concentration of the alloy, Curie temperature (TC) increases from ∼220 K (for x = 0) to ∼500 K (for x = 4). The maximum magnetic entropy change |ΔSM|max of the alloy, which is achieved near TC, is larger than 1.2 J kg−1 K−1 (under magnetic change ΔH = 12 kOe) for all the concentrations of Co. This has a significant meaning for magnetic refrigeration applications.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since its discovery, magnetocaloric effect (MCE) of material has been attracting many scientists because of the possibility of using it for magnetic refrigeration. The application of magnetocaloric materials in chillers has the advantages of not causing environmental pollution (unlike chillers using compression gases), capability of improving the cooling efficiency (saving energy), compact design, less noise and ability to be used in some special applications. Magnetic refrigeration is based on the principle of magnetic entropy change of the material. To have large magnetic cooling efficient, the MCE of the material should be large. There are three main factors to estimate potential magnetocaloric materials: the magnetic entropy change (ΔSM), the adiabatic temperature change (ΔTad) and refrigerant capacity (RC). An ideal magnetic refrigerant has to possess large values of both the ΔSM and RC around room temperature in low magnetic field.
Magnetocaloric materials are actually of interest to research nowadays due to new discoveries of both the mechanism and magnitude of MCE. The search for magnetocaloric materials with the possibility for applications in magnetic refrigeration at room temperature region are of more and more interest for scientific research. These materials include As-containing alloys (MnAsSb, MnFePAs, etc), La-containing alloys (LaFeSi), Heusler alloys (CoMnSi, NiMnSn, NiMnGa, etc), and ferromagnetic perovskite maganites [1–10]. Among these materials, amorphous magnetic or nanocrystalline materials are potential candidates [11–19] because they (i) undergo second-order magnetic phase transitions which present a broad ΔSM peak around the Curie temperature TC, (ii) possess large RC, (iii) do not exhibit structural changes [15] and (iv) have small magnetic and thermal hysteresis, high electrical resistivity and good mechanical properties [16]. Among this kind of materials, rare-earth-based amorphous alloys have high ΔSM peak and large RC. Particularly, LaFe13-based alloys have attracted intensive attention due to their low price, giant magnetocaloric effect (GMCE) and high thermal conductivity. But their Curie temperature TC is under room temperature, making them unsuitable for ambient-temperature applications [17–19]. To enhance the Curie temperature TC of this material, the addition of Co is the most suitable way. Moreover, Co can improve the glass-forming ability (GFA) of the materials.
In this work we replaced a part of Fe by Co in LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) alloy prepared by using melt-spinning method.
2. Experimental
Ingots with nominal compositions of LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) were prepared from pure components of La, Fe, Co and Si on an arc-melting furnace to ensure their homogeneity. The ribbons were then fabricated on a single wheel melt-spinning system. The quenching rate of the ribbons could be changed by changing tangential velocity, v, of the wheel. In this study, the ribbons were prepared with v = 40 m s−1. All the arc-melting and melt-spinning were performed under Ar atmosphere to avoid oxygenation. Structure of the ribbons was analyzed by x-ray diffraction (XRD). Magnetic measurements in the temperature range of 77–400 K were performed on a vibrating sample magnetometer (VSM).
The values of magnetic entropy change ΔSM caused by a variation of applied magnetic field was calculated by using the formula

3. Results and discussion
LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) alloy ribbons were prepared with v = 40 m s−1. This is the highest velocity of the wheel. With this quenching rate, the amorphous-to-crystalline transformation is the lowest during the quenching process. Figure 1 shows XRD patterns of LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) ribbons. We can see that all samples are partly crystallized. The results of structure analyses also show that the phase formation clearly depends on the concentration of Co. Intensities of the diffraction peaks are gradually reduced with Co addition. There are two diffraction peaks of the XRD patterns corresponding to α-Fe phase. Other peaks are those of cubic NaZn13-type phase [19].
Figure 1. XRD patterns of LaFe11−xCoxSi2 (x = 1, 2, 3, 4 and 5) alloy ribbons.
Download figure:
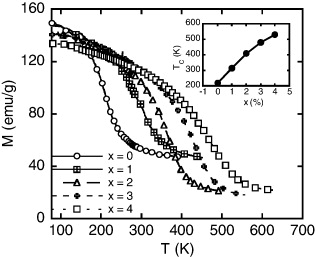
Standard image High-resolution imageFigure 2 represents thermomagnetization curves of the LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) alloy ribbons with an applied magnetic field of 12 kOe. We see that these ribbons have a magnetic phase transition in the range of 200–550 K. The magnetization decreases with increasing temperature and rapidly decreases at the magnetic phase transition. After the magnetic phase transition, the magnetization of all the ribbons does not reduce to zero but keeps at certain values characterizing for the crystalline phase. This is suitable for the above structure analysis. It should be noted that Co-concentration strongly influences the Curie temperature of LaFe11−xCoxSi2 (x = 1, 2, 3, 4 and 5) ribbons. The Curie temperature is enhanced with increasing Co-concentration (see inset of figure 2). Without Co-addition, the magnetic phase transition of alloy is below room temperature. The TC value determined for the sample x = 0 is only ∼220 K. However, when the concentration of Co is 1 at %, the phase transition temperature is raised to 315 K. As for the samples with x = 2, 3 and 4, the TC values are 410, 480 and 530 K, respectively (see table 1). The effect of Co-addition on Curie temperature has a significant effect in controlling the working temperature of the magnetic refrigerants.
Figure 2. Thermomagnetization curves in an applied magnetic field of 12 kOe of LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) alloy ribbons. The inset shows the Curie temperature TC versus Co concentration of these samples.
Download figure:
Standard image High-resolution imageTable 1. Influence of Co concentration on magnetization at 12 kOe (M12 kOe), Curie temperature (TC), maximum entropy change (|ΔSM|max), full-width at half-maximum (FWHM) of entropy change peak and refrigerant capacity (RC) of the LaFe11−xCoxSi2 (x = 0, 1 and 2) alloy ribbons (ΔH = 12 kOe).
| x (%) | M12 kOe (emu g−1) | TC (K) | |ΔSM|max (J kg−1 K−1) | FWHM (K) | RC (J kg−1) |
|---|---|---|---|---|---|
| 0 | 45 | 220 | 1.43 | 55 | 79 |
| 1 | 77 | 315 | 1.25 | 67 | 84 |
| 2 | 99 | 410 | 1.26 | 70 | 88 |
| 3 | 105 | 480 | – | – | – |
| 4 | 111 | 530 | – | – | – |
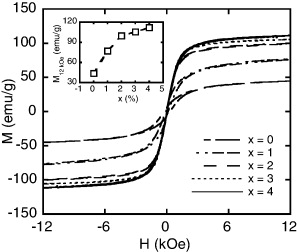
Figure 3 shows the dependence of magnetization on external field and Co-concentration of the ribbons at 300 K. From these hysteresis loops, both the saturation magnetization MS and the coercivity Hc were obtained. All the samples manifest the soft magnetic behavior and their magnetization increases with increasing Co-concentration. Co increases saturation magnetization of the alloy (inset figure 3). This is due to the formation of LaCo13 phase that increased with the addition of Co. The highest Ms value of the alloy is above 100 emu g−1 for x = 4. These two effects of Co furthermore improve capacity for application of the alloy in the magnetic refrigeration.
Figure 3. Hysteresis loops at 300 K of LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) ribbons. The inset shows magnetization at field of 12 kOe versus Co concentration of these samples.
Download figure:
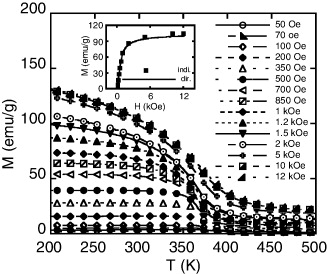
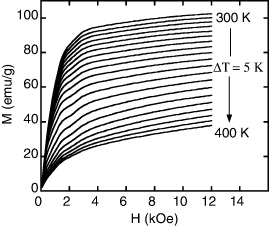
Standard image High-resolution imageWith the goal of finding magnetocaloric materials that possess magnetic transition temperature close to room temperature, we chose three samples of LaFe11−xCoxSi2 ribbons with Co concentration in the range of 0–2 at.% to investigate their MCE. We calculate magnetic entropy change (ΔSM) based on thermomagnetization data (figure 4). We can derive from thermomagnetization curves in different magnetic fields of the samples to be magnetized versus magnetic field curves at various temperatures (figure 5). To check this derivation procedure, we compared data (indirect data) deduced from thermomagnetization curve with data (direct data) of virgin magnetization ones (see inset of figure 4). We found that the data obtained from the two different ways coincided. Then the magnetic entropy change, ΔSM, is determined from M(H) data by using equation (1).
Figure 4. Thermomagnetization curves in various magnetic field of LaFe9Co2Si2 ribbon. The inset shows magnetization versus magnetic field at 300 K obtained from virgin magnetic (dir.) and thermomagnetization (ind.) curves.
Download figure:
Standard image High-resolution imageFigure 5. Magnetization versus magnetic field at various temperatures deduced from thermomagnetization curves of LaFe9Co2Si2 ribbon.
Download figure:
Standard image High-resolution imageBy using the proposed method to calculate the magnetic entropy change of magnetocaloric materials, we can save time and expenditure for experiments. This method is also useful to avoid the effect of thermal fluctuation of measurement systems.
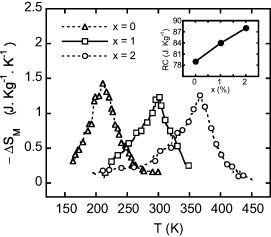
Figure 6 shows the plots of the temperature dependences of −ΔSM for the samples with x = 0, 1 and 2 in a field interval of 0–12 kOe. The results indicate that the maximum entropy change ( ) is nearly unchanged (above 1.2 J kg−1 K−1 with ΔH = 12 kOe) with various Co concentrations. However, working temperature of the alloy and FWHM of entropy change peak gradually increase with increasing Co concentration. The refrigerant capacity (RC) of the samples, which is defined as the product of maximum entropy change and FWHM of entropy change peak, was also calculated (see inset of figure 6). The
) is nearly unchanged (above 1.2 J kg−1 K−1 with ΔH = 12 kOe) with various Co concentrations. However, working temperature of the alloy and FWHM of entropy change peak gradually increase with increasing Co concentration. The refrigerant capacity (RC) of the samples, which is defined as the product of maximum entropy change and FWHM of entropy change peak, was also calculated (see inset of figure 6). The  and RC values determined for the samples with x = 0, 1 and 2 are 1.43, 1.25 and 1.26 J kg−1 K−1 and 55, 67 and 70 J kg−1, respectively (see table 1). One can see that the sample with Co concentration of 1 at.% has quite large
and RC values determined for the samples with x = 0, 1 and 2 are 1.43, 1.25 and 1.26 J kg−1 K−1 and 55, 67 and 70 J kg−1, respectively (see table 1). One can see that the sample with Co concentration of 1 at.% has quite large  and RC at around room temperature. This shows a capability to apply this kind of magnetocaloric materials in practice.
and RC at around room temperature. This shows a capability to apply this kind of magnetocaloric materials in practice.
Figure 6. ΔSM(T) curves (ΔH = 12 kOe) of LaFe11−xCoxSi2 (x = 0, 1 and 3) ribbons. The inset indicates refrigerant capacity (RC) versus Ni-concentration of the alloy.
Download figure:
Standard image High-resolution image4. Conclusion
The magnetic and magnetocaloric properties in LaFe11−xCoxSi2 (x = 0, 1, 2, 3 and 4) alloy ribbons have been studied. Glass forming ability of the alloy can be enhanced considerably by the addition of Co. Curie temperature of the alloy can be regulated in room temperature region by replacement of a part of Fe by Co. The quite high maximum magnetic entropy change (|ΔSM|max > 1.2 J kg−1 K−1 with ΔH = 12 kOe), large refrigerant capacity (RC > 80 J kg−1) and wide working range around room temperature (FWHM > 60 K) reveal application potential of this alloy in magnetic refrigeration technology.
Acknowledgments
This work was supported by the Scientific Research and Technology Development Project for Young Researchers of VAST, the National Foundation for Science and Technology Development (NAFOSTED) of Vietnam under grant number of 103.02–2011.23 and the Converging Research Center Program funded by the Ministry of Education, Science and Technology (2012K001431), South Korea. A part of the work was done in the Key Laboratory for Electronic Materials and Devices, and Laboratory of Magnetism and Superconductivity, Institute of Materials Science, VAST.