Abstract
Hesperetin (HET), a naturally occurring plant bioflavonoid present in citrus fruits, possesses potential anti-inflammatory and anti-carcinogenic activities but poor aqueous solubility limits its applications. To improve its applicability in cancer therapy, hesperetin was encapsulated in Eudragit® E (EE) 100 nanoparticles in the presence of polyvinyl alcohol (PVA) as a stabilizer and its anticancer efficacy in oral carcinoma (KB) cells was studied. Hesperetin-loaded nanoparticles (HETNPs) were prepared by nanoprecipitation method and characterized by dynamic light scattering (DLS), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy, differential scanning calorimetry (DSC), and x-ray diffraction (XRD). The results thus displayed that the prepared nanoparticles showed a particle size in the range from 55 to 180 nm. The encapsulation efficiency of hesperetin was 83.4% obtained by UV spectroscopy. The in vitro release kinetics of hesperetin under physiological condition show initial rapid release followed by slow and sustained release. 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay revealed higher cytotoxic efficacy of HETNPs than native hesperetin in KB cells. Further, it has been found that reactive oxygen species (ROS) generation, DNA damage and apoptotic indices in HETNPs treated cells are greater than those in native hesperetin treatment. Hence these findings demonstrate that HETNPs could be a potentially useful drug delivery system to produce better hesperetin therapeutics of cancers.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Flavonoids are the most abundant polyphenols present in the human diet and are components found in vegetables, fruits and plant-derived beverages such as tea and red wine [1]. Flavonoids are composed of several classes including flavonols, flavonones, flavones, flavanols, iso-flavonoids and antho-cyanidins [2]. Epidemiology and animal studies have suggested that a high intake of flavonoids may be linked to a reduced risk of cancer. Hesperetin (HET) (3',5,7-trihydroxy-4-methoxyflavonone), one of the most abundant flavonoids found in citrus fruits which occurs as hesperidin (its glycoside form) in nature and it has received considerable attention in cancer prevention. It exhibits various pharmacological activities, such as anti-inflammatory, anti-hypertensive and anti-atherogenic effects [3–5]. Recently, growing evidence on the therapeutic efficacy of hesperetin has been established in preclinical models (principally, in cell lines) derived from a variety of solid tumors [6–9].
Despite the promising application of hesperetin in cancer therapy, but the clinical use of hesperetin was restricted because of the poor water solubility. Thus a novel aqueous formulation of hesperetin was desirable. Therefore, many researchers are now focusing on improving its bioavailability through several approaches including innovative drug delivery systems (β-cyclodextrin complex, phospholipids complex and nanoformulation) [10–12]. Among them, nanoformulation holds greatest promise for increasing bioavailability. The nanoparticles showed advantages such as more stability during storage over others. Moreover, such a colloidal system is able to extravasate solid tumors into the inflammed or infected site, where the capillary endothelium is defective [13]. Encapsulation of hydrophobic drugs into nanoparticles can make the drug completely dispersible in water, making the drug release in a controlled and sustained manner.
The development of polymeric nanoparticles has been attracting considerable interest in recent years due to their promising applications in cancer therapy [14–16]. Polymeric nanoparticles act as nanocarriers with many advantages, such as low toxicity and high stability [17]. This could avoid recognition by the P-glycoprotein (P-gp) efflux pump and, thus, have a strong potential to enhance the oral bioavailability of poorly absorbed drugs [18]. Their small size and their large specific surface area favor their absorption compared to larger drug carriers [19]. The enhanced permeability and retention (EPR) effect of polymeric NPs near the tumor vasculature helps in passive targeting of anticancer drugs [20].
In the preparation of drug nanoparticles system, polymers are required for the immediate entrapment of the free compound and are used as carriers. Eudragit® E (EE) is a cationic copolymer which has been widely used to improve the solubility of poorly water-soluble drugs [21]. It has a basic site containing tertiary amine groups which are ionized in gastric fluid and, therefore, it is easy to dissolve in the gastric environment [22]. Recent studies reported that some new nanoparticle formulations of naringenin-loaded EENPs, kaempferol-loaded EENPs and resveratrol loaded EENPs prepared by nanoprecipitation method using PVA as stabilizer and the efficacy of these nanoformulations were observed to be more effective than the native drug [23–25]. Despite a lot of information on the usage of HET in anticancer studies, the implementation of drug at the nanoscale in cancer cell lines has not been studied so far. Therefore, in the present study hesperetin-loaded Eudragit® E nanoparticles (HETNPs) were developed and to study the anticancer potency in oral carcinoma (KB) cells. Physicochemical properties of prepared nanoparticles were studied by dynamic light scattering (DLS), transmission electron microscope (TEM), Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimeter (DSC) and x-ray diffraction (XRD). Encapsulation efficiency and in vitro drug release pattern was studied by UV spectrophotometer. Furthermore, the anticancer effect of HETNPs was also determined by investigating the effect on cell viability, intracellular ROS, cell morphology, mitochondrial membrane potential and DNA damage in oral carcinoma (KB) cells.
2. Experimental
2.1. Chemicals
Hesperetin, polyvinyl alcohol (MW 25 000), 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT), 2',7'-dichlorofluoresein diacetate (DCFH-DA), heat-inactivated fetal calf serum (FCS) and Dulbecco's modified eagle medium (DMEM), antibiotics pencillin–streptomycin were obtained from Sigma-Aldrich Chemical Pvt Ltd, Bangalore, India and used as received. Acridine orange (AO), ethidium bromide (EB), rhodamine 123 (Rh123) and phosphate buffered saline (PBS) were purchased from Himedia, Mumbai. Aminoalkyl methacrylate copolymer Eudragit® E 100 (EE 100) was a kind gift sample supplied by Evonik Industries (Mumbai, India). All other used chemicals and solvents used were of analytical grade.
2.2. Cell culture and maintenance
Human oral carcinoma (KB) cell lines were obtained from National Centre for Cell Science (NCCS), Pune, India. The cells were cultured in DMEM with 10% FCS, 100 U ml−1 of penicillin and 100 μg ml−1 of streptomycin at 37 °C in a humidified 95% air and 5% CO2 incubator.
2.3. Preparation of hesperetin-loaded nanoparticles (HETNPs)
The hesperetin nanoparticles systems with a ratio of HET:EE:PVA of 1:10:10 (w/w/w) were prepared by the nanoprecipitation method [24]. Briefly, 200 mg of EE 100 was added to 10 ml of ethanol and dissolved in a water bath at 37 °C. Next, 20 mg of hesperetin was added to the organic solution. The organic phase solution was then quickly injected into a 30 ml external aqueous solution containing 200 mg of PVA and homogenized at 18 000 rpm for 40 min. Subsequently, the ethanol was removed by rotary vacuum evaporation in a 40 °C water bath. The remaining solution, the hesperetin nanoparticles (HETNPs) solution, was stored in a refrigerator until being used for anticancer activity assays. Some of the HETNPs were lyophilized with a freeze-dryer, and the lyophilized powder was collected and stored in a moisture-proof container until further studies.
2.4. Characterization of HETNPs
2.4.1. Particle size and zeta potential measurements.
Particle size and size distribution of HETNPs were determined by nanotrac (Microtrac, Inc., USA) based on the principle of DLS. For the above measurement, the lyophilized nanoparticles were suspended in double-distilled water and sonicated to get a homogeneous suspension. The size measurement was performed in triplicate.
The zeta potential of HETNPs was measured by Malvern Zetasizer ZS (Malvern Instruments, Malvern, UK). The measurement of zeta potential is based on the direction and velocity of particles under the influence of known electric field.
2.4.2. Transmission electron microscopy (TEM).
The size and morphology of HETNPs was observed by TEM. A drop of dilute sonicated solution was negatively stained with 1% (w/v) uranyl acetate for 10 min and deposited onto the carbon-coated copper TEM grid and was allowed to air dry. The samples were imaged using a JEOL-2100 transmission electron microscope with 100 kV.
2.4.3. Fourier transform infrared spectroscopy (FT-IR) study.
FT-IR spectra were taken on a Nicolet Avatar 330 FT-IR spectrometer to investigate the possible interaction between native hesperetin, blank nanoparticles and HETNPs. Briefly, ∼5 mg of powdered samples were gently mixed with KBr (at a ratio of 1:10) to obtain pellets using a hydraulic press. FT-IR spectra were scanned in the range of 4000 and 400 cm−1 at a resolution of 4 cm−1.
2.4.4. Differential scanning calorimeter (DSC) analysis.
DSC was performed to investigate the physical state of the HET inside HETNPs. The DSC curves were obtained by DSC thermogram analysis (DSC Q20 V 24.2 Build 107). For analysis, ∼10 mg of lyophilized samples were placed in aluminum sample cells and were heated at the rate of 10 °C min−1 under the flow of nitrogen gas at an outlet pressure of 6–10 kg cm−2. The heat flow was recorded from 40 to 300 °C.
2.4.5. X- ray diffraction (XRD) analysis.
X-ray diffraction analysis of the native HET and were recorded using x-ray diffractometer (X'Pert PRO-PANalytical Philips) at λ = 0.1546 nm, running at 40 kV and 30 mA. Diffraction patterns were obtained in the region from 10° to 80° at a scan speed of 2θ per minute.
2.4.6. Determination of encapsulation efficiency (EE).
A weighed amount of dried HETNPs were dissolved in distilled water and then centrifuged, followed by the collection of the supernatant. The absorbance of the supernatant solution was measured by UV spectrometer (UV-1800 Shimadzu) at the wavelength of 288 nm and the weight of the drug loaded was calculated using calibration curve. The EE was calculated from the following formula:
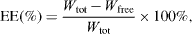
where Wtot is the total weight of HET in HETNPs, Wfree is the free HET released from HETNPS.
2.4.7. In vitro drug release experiment.
HETNPs (5 mg) was suspended in 100 ml of phosphate-buffered saline solution (PBS, pH 7.4) at 37 °C and placed in a water bath shaker with a thermostat at 120 rpm. At predetermined intervals, 3 ml of aliquots was withdrawn and replaced by 3 ml of fresh PBS to maintain the sink conditions. The concentration of the released drug was determined by UV spectrometer (UV-1800 Shimadzu) at 288 nm:
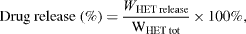
where WHET release is the amount of hesperetin releasing from HETNPs, WHET tot is the total amount of hesperetin in HETNPs.
2.4.8. Cytotoxicity assay.
Cytotoxicity of native hesperetin and hesperetin-loaded nanoparticles on oral carcinoma (KB) cell line was analyzed by the MTT calorimetric assay, based on the cleavage of a yellow tetrazolium salt to insoluble purple formazan crystals by the mitochondrial dehydrogenases of viable cells. KB cell lines were seeded in 96 well plates at the density of 8000 cells per well and kept in an incubator up to 24 h for acclimatization. After 24 h, cells were treated with various concentrations (10, 20, 40, 60 and 80 μg ml−1) of native hesperetin and HETNPs. Cells were incubated in a CO2 incubator at 37 °C for 48 h. After 48 h of incubation, the medium was removed and 100 μl of fresh medium was added along with (5 mg ml−1) MTT solutions and incubated for another 4 h in a CO2 incubator. The extent of cell viability was determined by the conversion of yellow MTT into purple formazan by the living cells [26]. After the medium was aspirated, the formed formazan crystals were dissolved in 200 μl of dimethylsulfoxide (DMSO) and its absorbance was measured at 540 nm using a microplate reader. The half maximal inhibitory concentration (IC50) values were calculated and the optimum dose was used for further study.
2.4.9. Measurement of intracellular reactive oxygen species (ROS).
The measurement of intracellular ROS formation was based on the oxidative conversion of 2',7'-dichlorofluorescein-diacetate (DCFH-DA) to fluorescent compound dichloroflouorescin (DCF) [27, 28]. Briefly, after treatment, KB cells were harvested and suspended in 0.5 ml PBS containing 10 μl DCFH-DA for 15 min at 37 °C in the dark. DCFH-DA was taken up by the cells and deacetylated by cellular esterase to form a non-fluorescent product DCFH, which was converted to a green fluorescent product DCF by intracellular ROS produced by treated KB-cells. Fluorescent measurements were made with excitation and emission filters set at 488 and 530 nm, respectively. Fluorescence microscopic images were taken using blue filter (450–490 nm) (Nikon, Eclipse TS100, Japan).
2.4.10. Analysis of mitochondrial membrane potential (MMP, Δψm).
Alteration in mitochondrial membrane potential is an indication of early stages of apoptosis [29]. Rhodamine 123 (Rh-123) is a lipophilic cationic dye, highly specific for mitochondria. Rh-123 dye was used to detect changes in mitochondrial membrane potential on the HET and HETNPs treated cells. After treatment with IC50 concentration of native HET and HETNPs for 48 h, fluorescent dye Rh-123 (10 μg ml−1) was added to the cells and kept in incubation for 30 min. Then, the cells were washed with PBS and viewed under fluorescence microscope using blue filter.
2.4.11. Apoptotic morphological changes by AO/EB staining.
DNA-binding dyes AO and EB were used for the morphological apoptotic and necrotic cells [30]. AO is taken up by both viable and non-viable cells and emits green fluorescence if intercalated into double stranded nucleic acid (DNA). EB is taken up only by non-viable cells and emits orange red fluorescence by intercalation into DNA. After treatment with IC50 dose of HET and HETNPs for 48 h, the cells were detached, washed by cold PBS and then stained with a mixture of AO (100 μg ml−1) and EB (100 μg ml−1) at room temperature for 5 min. The stained cells were observed by a fluorescence microscope at 40 × magnifications.
2.4.12. Measurement of oxidative DNA damage.
DNA damage was estimated by alkaline single cell gel electrophoresis (comet assay). The extent of DNA damage was estimated by fluorescence microscope using a digital camera and analyzed by image analysis software, CASP. For each sample, 100 comet images were analyzed and tail moment, tail length and olive tail moment were quantified [31].
2.4.13. Statistical analysis.
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) by using statistical package of social science (SPSS) version 11.0 for windows. The values are ±SD for six samples in each group. Values of p < 0.05 were considered as level of significance.
3. Results
3.1. Physicochemical characterization of hesperetin loaded nanoparticles
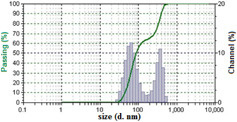
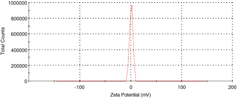
Hesperetin loaded nanoparticles were prepared from Eudragit® E copolymer using nanoprecipitation method. The functional performance of nanoparticles based delivery systems depends on the physicochemical properties of the nanoparticles, such as size, morphology and physical state. Dynamic light scattering analysis revealed that the formulated nanoparticles had an average size of 180 nm (figure 1) with slight positive potential (+ 0.962 mV) (figure 2).
Figure 1. Mean particle size of hesperetin-loaded nanoparticles (HETNPs) measured by dynamic light scattering.
Download figure:
Standard image High-resolution imageFigure 2. Zeta potential distribution of HETNPs.
Download figure:
Standard image High-resolution imageTEM studies were performed to further confirm the size and morphology of the HETNPs. TEM images (figure 3) showed the separate particles with spherical shape and size distribution (∼ 55 nm) of HETNPs. The hydrodynamic size of NPs achieved by DLS analysis as compared to the size obtained by TEM analysis may be contributed by the hydration of the surface associated PVA. Moreover, from the UV spectral analysis, it was observed that hesperetin was efficiently loaded in NPs, reaching a high encapsulation efficiency of 83.4 ± 2.1%. This implies that ∼83.4% of the drug was entrapped inside the nanoparticles. Further, FT-IR analysis was used to characterize any chemical interaction that occurred in the polymer due to the addition of the drug during NP formulation. Figure 4 shows the FT-IR spectra of blank nanoparticles, native hesperetin and HETNPs. The spectra of HETNPs showed the presence of bands similar to blank NPs along with some extra bands due to the entrapment of drug. The FT-IR spectra of native hesperetin illustrated characteristic bands due to different functional groups such as 3495, 1637, 1509 and 1024 cm−1, corresponding to  stretching vibration, CONH amide I stretching, aromatic
stretching vibration, CONH amide I stretching, aromatic  stretching and
stretching and  stretching, respectively. However, the vibrational spectra of native HET at 3495, 2909, 2734, 1263 cm−1 are slightly shifted to 3423, 2923, 2767, 1272 cm−1 in HETNPs due to some minor chemical interaction between native hesperetin and Eudragit matrix.
stretching, respectively. However, the vibrational spectra of native HET at 3495, 2909, 2734, 1263 cm−1 are slightly shifted to 3423, 2923, 2767, 1272 cm−1 in HETNPs due to some minor chemical interaction between native hesperetin and Eudragit matrix.
Figure 3. Transmission electron microscopic (TEM) images of HETNPs.
Download figure:
Standard image High-resolution imageFigure 4. The FT-IR spectra of blank nanoparticles, native HET and HETNPs.
Download figure:
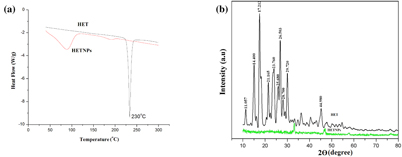
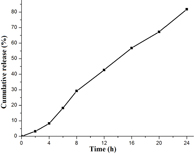
Standard image High-resolution imageDSC and XRD studies are carried out to determine whether the drug was incorporated in the nanoparticulate system as crystalline, amorphous, or bound form. The DSC thermograms demonstrated that only native hesperetin had an endothermic peak of melting point at 230 °C, whereas hesperetin-loaded nanoparticles had no such peak, which showed encapsulated HET to be in either amorphous form or in disordered crystalline phase (figure 5(a)). Furthermore, x-ray diffraction patterns of native HET and HETNPs are present in figure 5(b). Native HET has displayed the characteristic crystalline peaks of 2θ value of 14.400°, 17.212°, 21.165°, 23.760°, 26.503° and 29.720°. However, no characteristic crystalline peaks were observed when the drug was encapsulated in NPs (figure 5(b)). This indicates that the drug was molecularly dispersed or in an amorphous state, which favors easy diffusion of drug molecules through the polymeric matrix, resulting in sustained release of the drug from the nanoparticles. Sustained release of the drug from nanoparticles is an important parameter for developing successful formulations. HETNPs showed a biphasic drug-release pattern that was characterized by an initial rapid release of approximately 29.1% of drugs in the first 8 h, followed by a slow and continuous release of approximately 81.8% drug release in the next 24 h (figure 6). The observed initial release of hesperetin might be due to a rapid release of some drug loosely bound on the surface of the nanoparticles by a mechanism of diffusion. The initial rapid release was followed by a slower sustained release of hesperetin present inside the core of the nanoparticles. A similar trend of release was observed by our previous studies [23].
Figure 5. (a) DSC endothermic curve of native HET and HETNPs and (b) x-ray diffraction (XRD) patterns of native HET and HETNPs.
Download figure:
Standard image High-resolution imageFigure 6. Cumulative in vitro release profile of native HET from nanoparticles.
Download figure:
Standard image High-resolution image3.2. In vitro cytotoxicity studies
To investigate the therapeutic efficacy of hesperetin, KB cells were treated with native hesperetin and HETNPs at different concentrations for 48 h and cell proliferation was measured by a standard MTT calorimetric assay. As can be seen from figure 7, HETNPs demonstrated higher anti-proliferative activity than native hesperetin. Observation from IC50 values indicated 2.3 times superior anti-proliferating efficiency for HETNPs (35.4 μg ml−1) than that of native hesperetin (78.6 μg ml−1) and it was used for further experiments.
Figure 7. Dose dependent cytotoxicity of native hesperetin and HETNPs.
Download figure:
Standard image High-resolution image3.3. Role of reactive oxygen species in hesperetin-loaded nanoparticles (HETNPs)
ROS is an important parameter and it can be capable of producing free radicals and induced severe cell death compared to normal cells. In the present study, intracellular ROS levels were detected by fluorescent H2DCF-DA dye in native HET and HETNPs. As shown in figure 8(a), treatment of cells with HETNPs showed bright DCF fluorescence compared to native hesperetin. Further, spectrofluorimetric analysis also confirms the increased intracellular ROS generation during HETNPs treatment over native HET (figure 8(b)).
Figure 8. (a) Fluorescence microscopic images of intracellular reactive oxygen species (ROS) measurement by DCFH-DA staining. Arrow mark (→) represents high DCF fluorescence in HETNPs treatment and (b) intracellular ROS measurement by spectrofluorimeter.
Download figure:
Standard image High-resolution image3.4. Effect of HETNPs on mitochondrial membrane potential (MMP, Δ□m)
Loss of MMP has been shown to be an early event during apoptosis in some systems. Mitochondrial membrane potential changes, as measured by Rh-123 fluorescence in native HET and HETNPs for 48 h, are shown in figure 9(a). Treatment with HETNPs resulted in the increased depolarization of the MMP as revealed by changes in Rh-123 fluorescence dye from orange red to green, compared to native HET and control. Further, HETNPs treatment showed elevated fluorescence intensity for Rh-123 absorption (figure 9(b)) indicates increased mitochondrial depolarization compared to native HET.
Figure 9. (a) Fluorescence microscopic images of mitochondrial membrane potential by Rh-123 staining and (b) altered MMP by spectrofluorimeter.
Download figure:
Standard image High-resolution image3.5. Effect of HETNPs on apoptotic morphological changes
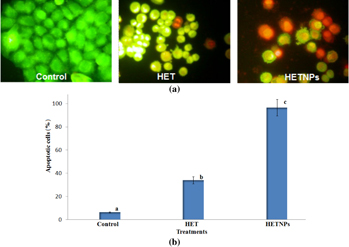
Morphological features of apoptosis such as chromatin condensation, nuclear fragmentation, alterations in the size and the shape of cells, as revealed by fluorescence microscopic analysis. Staining with acridine orange (AO) and ethidium bromide (EB) showed significant fragmentation and condensation of chromatin in KB cells treated with native HET and HETNPs for 48 h (figure 10(a)). While the control cells exhibited a normal nuclear morphology characterized by a diffused chromatin structure and, therefore, light green staining (figures 10(a) and (b)) shows the quantitative result of apoptosis in the control, native HET and HETNPs treatment.
Figure 10. (a) Fluorescence microscopic images of apoptotic morphology by dual staining and (b) percentage of apoptosis.
Download figure:
Standard image High-resolution image3.6. Effect of HETNPs on DNA damage in KB cells
Figure 11(a) shows the photomicrograph of DNA damage (comet) in KB cells. The control cells showed largely non-fragmented intact nucleoid. The tail DNA was observed in HET and HETNPs treated cells which appeared as a comet during single cell gel electrophoresis. Treatment with HET and HETNPs resulted in an increase in DNA damage. Figure 11(b) shows the changes in the levels of DNA damage (% DNA in tail, tail length, tail moment and olive tail moment) in control, HET and HETNPs treated in KB cells. The result shows no changes in the levels of DNA damage in the untreated control cells. HETNPs treatment caused significantly increased % tail DNA, % tail length, % tail moment and % olive tail moment when compared to HET treatment alone.
Figure 11. (a) Photomicrographs of DNA damage (comet assay) and (b) percentage of tail length, tail DNA, tail moment and olive tail moment.
Download figure:
Standard image High-resolution imageIn figures 8–11 the values are given as a mean ±SD of six experiments in each group (one-way analysis of variance followed by Duncan's multiple range test). Values that are not sharing the common superscripts differ significantly (p < 0.05).
4. Discussion
The usage of anticancer drugs is limited by short plasma half-life and common side effects such as systemic toxicity due to high dosage and no specificity with respect to healthy cells and low bioavailability. Nanoparticles possess the ability to permeabilize the cells more efficiently than microspheres due to their smaller size, which facilitates administration of large quantities of drug to give better efficacy. Encapsulation of anticancer drugs in polymeric nanoparticles could potentially overcome both P-gp mediated drug efflux and first pass metabolism [18].
In the present study, hesperetin-loaded nanoparticles were prepared by nanoprecipitation method and its anticancer efficiency in KB oral cancer cells was tested. Particle size of nanoparticles plays a crucial role in their antitumor activity and in vivo distribution [32, 33]. Smaller nanoparticles show a higher accumulation at tumor sites and prolong in vivo half-life due to their avoidable capture by the reticuloendothelial system [34, 35]. From the results of DLS technique, the average particle size is nearly 180 nm. TEM results clearly indicated that particles have nearly spherical shape with an average size of ∼55 nm. The size measured by DLS is that of water-swollen particles (hydrodynamic diameter), whereas the size deduced from TEM measurement corresponds to particles in a dried state. Therefore, the particle size from TEM measurements in a dried state cannot be compared with the values from DLS measurements and DLS gave higher values compared to TEM [36]. Particle size is controlled around 180 nm which is in favor of the antitumor activity and prolongs efficacy of HETNPs. Nanoparticles with small particle size (< 200 nm) are reported to have cross vasculature endothelia and accumulated at tumor sites via the enhanced permeation retention (EPR) effect [23]. It was further established that the optimal particles size is ranging from 20 to 400 nm [37]. Hesperetin-loaded nanoparticles are thus convenient to benefit from the EPR effect. Further, the zeta potential values are close to zero. The factor which might be responsible for such an effect can be the presence of residual PVA on the nanoparticles surface.
Characterization of the physiochemical properties of the drug encapsulated within the nanoparticles may possibly reveal useful information for the feasibility of using nanoparticles for cancer therapy. FT-IR results confirmed that drug is encapsulated inside the polymer. DSC results of HET showed a sharp endothermic peak at 230 °C which is the well known melting point of hesperetin, but in the case of HETNPs the melting peak totally disappeared evidencing the absence of crystalline drug in the nanospheres samples, at least at the nanosphere surface level. Therefore, it could be concluded that hesperetin in nanospheres was in an amorphous or disordered crystalline phase of molecular dispersion or a solid solution state in the polymer matrix after the production [38]. Further, this result is very well supported by XRD. Both DSC and XRD results confirmed that HETNPs have amorphous form. Encapsulation efficiency of hesperetin has increased because the hydrophobic portion of PVA interpenetrated into Eudragit chains during nanoprecipitation and remained to be trapped to the polymeric matrix of the nanoparticles. Accordingly, the addition of PVA easily formed an interconnected network with Eudragit-hesperetin and thus elevated the encapsulation efficiency of the drug. In the current study, the initial rapid release of hesperetin from nanoparticles was probably attributed to either the surface-bound moieties or an aqueous environment allowing increased water penetration. Afterwards, the HETNPs manifested the sustained release characteristics that appeared to be dependent on the hydrophobicity of hesperetin incorporated in the nanoparticles. The release pattern of the drug from the nanoparticles shows that long and continuous exposure of the cancer cells to anticancer drugs of a relatively lower and safer concentration gives little chance for the tumor blood vessels to grow, thereby resulting in much better efficacy [39]. Native HET and HETNPs were further evaluated for their in vitro cellular viability assay on KB cell lines by MTT assay. MTT results showed a good discrimination in cell inhibition between native HET and HETNPs, thus illuminating the key role of NPs binding and internalization in enhancement of cytotoxic activity. Encapsulation of anti-cancer drug in nanoparticles can increase its internalization by cells into lysosomes and enhanced cytotoxicity. The higher cytotoxic effect of hesperetin-loaded nanoparticles could be attributed to the competent cellular uptake and intracellular distribution of hesperetin.
In previous reports [40], anticancer drugs kill their target cells at least in part through the generation of intracellular ROS. The changes of ROS generation strongly influenced cell proliferation and caused apoptosis in cells incubated with NPs [41]. HETNPs treatment caused a rapid increase of intracellular ROS in KB cells compared to native HET. Due to the larger surface-area-to-mass ratio of HETNPs, more ROS could be formed than in native HET [42, 43]. Further, the changes in the mitochondrial membrane potential in native HET and HETNPs treated cells are observed. Mitochondria act as a point of integration for apoptotic signals originating from both the extrinsic and intrinsic apoptotic pathways. Mitochondria, which play a pivotal role in apoptosis, are major sites of ROS generation. Excessive generation of ROS can lead to opening of the mitochondrial permeability transition pore with decline in Δ□m and consequent release of cytochrome c from the intermembrane space into the cytosol culminating in activation of the caspase cascade and apoptotic cell death [44]. The disruption of MMP following HETNPs treatment in KB cells can be attributed to the changes in fluorescence from orange-red to green (figure 9(a)). The observed changes in the MMP could also be attributed to sustained release behavior of the nanoparticulate system.
Apoptosis is a gene-regulated phenomenon induced by many chemotherapeutic agents in cancer treatment and the characters of apoptosis including chromatin condensation, cell and nuclear shrinkage, membrane blebbing and oligonucleosomal DNA fragmentation [45, 46]. Among various methods used to detect apoptosis, chromatin condensation and nuclear fragmentation remain the hallmarks of apoptotic cells, since it allows distinguishing viable, early or late apoptotic and necrotic cells. In AO/EB double staining, AO permeates all cells and makes the nuclei appear green, whereas EB stains the nucleus red only when cytoplasmic membrane integrity is lost. Thus, live cells show a normal green nucleus, early apoptotic cells show a bright green nucleus with condensed or fragmented chromatin, late apoptotic cells show condensed and fragmented orange chromatin and cells that have died from direct necrosis show a structurally normal orange nucleus [30, 47]. In the present study, AO/EB staining also exhibited a higher number of apoptotic cells on treatment with HETNPs compared with native HET and control (figure 10(a)). The increased ROS levels and changes in the MMP might be the reason for the increased apoptotic morphological changes. It might also be due to the better uptake of nanoparticulate HET which resulted in greater accumulation of delivered HET inside cancer cells accompany by its sustained release, exerting a higher percentage of cells in apoptotic phase.
Comet assay, with the advantage of high sensitivity to single strand break, is suitable for assessing the effect of induced DNA damage. The induction of DNA single strand breaks is often used to predict the tumor cells. In the present study, the extent of DNA damage was greater in the HETNPs compared to native HET. Enhanced ROS generation during levels in HETNPs might be the reason for enhanced DNA damage. The results of the present study showed that treatment of HETNPs produced a dose-dependent cytotoxicity, alterations in mitochondrial membrane potential, apoptotic morphological changes and DNA damage indicating that HETNPs show obvious advantages over native HET in KB cells.
5. Conclusion
Hesperetin was successfully encapsulated on Eudragit® E 100 nanoparticles in the presence of PVA as a stabilizer by nanoprecipitation method. The prepared nanoparticles were characterized by DLS, TEM, FT-IR, DSC and XRD. The DLS and TEM results confirmed that the particle size was in the range of 55–180 nm. The FT-IR, DSC and XRD analyses clearly demonstrated that drug was encapsulated inside the polymer. The biphasic release profile includes initial rapid effect followed by sustained slow release. Moreover, the results of the present study conclude that treatment of HETNPs produces a dose-dependent cytotoxicity, increased intracellular ROS, alterations in mitochondrial membrane potential, apoptotic morphological changes and DNA damage. High encapsulation efficiency, small particle size and slow release make HETNPs a potentially useful drug delivery system for further development in in vivo studies.
Acknowledgment
The authors are grateful to the authority of Annamalai University for providing all necessary facilities to carry out the present study.