Abstract
A simple, single-step, one pot method was developed for the synthesis of monodispersed, ultralow sized, water-dispersible, stable, positively charged gold nanoparticles using the branched polyethlyneimine (PEI) in an aqueous media. Sizes of the gold nanoparticles have been tuned by adjusting the concentration of PEI and the gold salt. Formation of gold nanoparticles has been evidenced using various characterization techniques such as quasi-elastic light scattering (QELS), transmission electron microscopy (TEM), x-ray diffraction (XRD), energy dispersive x-ray (EDX) and spectroscopy and UV-visible spectrophotometer.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Gold nanoparticles are of increasing interest in materials chemistry due to the versatility in their physical and chemical properties. The research related to gold nanoparticles and its applications is becoming an inevitable area of interdisciplinary research. It has been established that these particles are highly water dispersible and remain suspended in water with no alteration of chemicals or physical properties over extended periods of time. At the nanoscale level, gold nanoparticles exhibit distinct colored appearance and they are reported to provide many potential applications in nano electronics, nanodevices, chemical separations, bio and chemo sensing, molecular marker, catalysis, coating decorative, optical materials, biosciences and drug delivery etc [1–7].
There are various methods for synthesizing gold nanoparticles, such as arc discharge [8], two phase synthesis [9], one phase synthesis in organic solvent [10], photochemistry [11, 12], reverse micelles [13–15], radiolysis [16], electrochemical methods [17–20], and sonochemical methods [21, 22]. A relatively recent development has been the use of the reducing agent which also acts as the 'nanoparticle capping species', and this technique has been demonstrated using amino acids [23–26]. Nevertheless, these above methods required sophisticated apparatus, which severely limits large scale production. Additionally, previous work used expensive surfactants (i.e. benzyl mercaptan, cetyl trimethyl ammonium bromide (C-TAB), sodium bis(2-ethylhexyl) sulfonate (AOT) as stabilizers for preventing aggregation of synthesized gold nanoparticles. The aforementioned methods also needed further purification steps [27–30].
In addition, gold nanoparticles were synthesized using different reducing agents (ascorbic acid, oxalic acid, or hydrazine) [31]. A simple aqueous reduction method has been shown in the past to synthesize negatively charged gold nanoparticles. The process is environmentally friendly and uses a simple apparatus for preparing gold nanoparticles and its derivatives [32, 33].
In contrast, we disclose a simple green approach to synthesize positively charged gold nanoparticles. We report a one-pot methodology to synthesize highly stable, charge/size tunable gold nanoparticles in aqueous solutions using polyethlyneimine (PEI) as a reducing agent (and a particle stabilizer) in this work. The branched nature, high electrical conductivity, reducing as well as stabilizing ability make it an excellent choice for the synthesis of gold nanoparticles for various biomedical and biochemical applications. In this work different parameters such as concentration of gold salt and PEI in the water varied to synthesize gold nanoparticles of different shapes, size distribution and stability. These results are anticipated to be of use in the synthesis of biocompatible and sensing materials for biomedical and chemical catalysis applications. We have demonstrated the use of gold nanoparticles as a vehicle for liver-specific drug delivery in the past [34], and have used nanoparticles for catalyzing diverse organic reactions [35–48].
2. Experimental
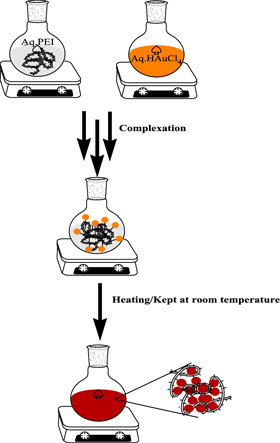
The methodology for the preparation of the gold nanoparticles using polyethlyneimine has been developed, where the PEI acts as stabilizing as well as reducing agent. The method is robust, reproducible and simple. Using the developed methodology one can synthesize gold nanoparticles either at room temperature in a stipulated time period or instantly by mild heating (scheme
Scheme 1. Schematic representation for the formation of gold nanoparticles.
Download figure:
Standard image High-resolution imageThe absorption spectrum of gold nanoparticles was recorded with a Hitachi AU-2700 spectrophotometer. The synthesized gold nanoparticles were dispersed in double-distilled water (1 ml), out of this sample solution (10 μl) was put on a formvar (polyvinyl formal) coated copper grid and then left overnight for air-drying. TEM pictures were taken with a model electron microscope (FEI Technai Ultra Twin 300 kV). Prior to visualization of samples, a blank grid without sample was also scanned. The average particle diameter of the prepared nanoparticles was analyzed by dynamic light scattering Instrument (Photocor FC, USA). Data analysis was performed with Alango dynal V 2.0 software.
Wide angle x-ray diffraction (XRD) patterns were obtained for gold nanoparticles by using Philips PW 1830 x-ray VB equipped with a 2 θ compensating slit, Cu-Kα radiation (1.54 Å) at 40 kV, 40 mA passing through Ni filter with a wavelength of 0.154 nm at 20 mA and 35 kV. Data collection was made in a continuous scan mode with a step size of 0.01° and step time of 1 s over a of 2 θ range of 10° to 80°. Data analysis was performed with PC-APD diffraction software.
3. Results and discussion
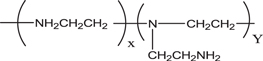
PEI is a highly branched, aliphatic, water soluble polyamine whose amine groups exist in primary, secondary and tertiary forms (scheme
Scheme 2. Chemical structure of polyethlyneimine.
Download figure:
Standard image High-resolution imageIt is well known that PEI can easily protonate in acid, even in neutral aqueous solution. PEI reduces AuCl4 −, causing it to nucleate and gradually leading to the formation of gold nanoparticles in the reaction
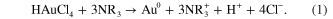
During the mixing of oxidative HAuCl4 solution with PEI, both protonate amine and neighboring methylene of PEI dehydrogenated to form a labile double bond (–C=N–) intermediate, which became amide at an elevated temperature. To gain an insight into the formation kinetics of gold nanoparticles, an in situ UV–Vis experiment was performed at a constant temperature of 60 °C (figure 1). In less than 30 s, the onset of formation of nanoparticles was confirmed with the appearance of a surface band centered at 515 nm. Evidently, the intensity of the absorption peak increases significantly in the following minute and then the rate of increase of the peak intensity decreases with elapsing time, possibly because of the increase of the concentration of reduced gold. It is also evidenced that the absorption band shifts continuously to longer wavelength at longer reaction time and ceases at the absorption band centered at 532 nm. This change occurs within an hour which may probably be explained on the basis of particle growth and Ostwald ripening. After this, no change in the absorption spectroscopy is noticed. The reduction of HAuCl4 occurs due to transfer of electrons from the amine to the metal ion, resulting in the formation of Au0 according to the reaction (1). The resulting metallic gold then undergoes nucleation and growth to form gold nanoparticles. The reducing potentials of the system indicates that the reduction is thermodynamically favorable and thus PEI reduces gold salt efficiently to form gold nanoparticles.
Figure 1. UV absorption spectra of in vitro synthesis of gold nanoparticles.
Download figure:
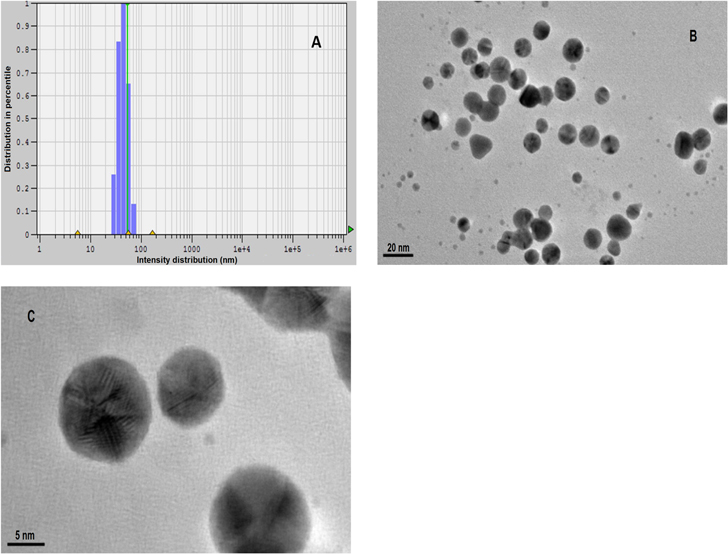
Standard image High-resolution imageResults of TEM analysis (figure 2) and the quasi-elastic light scattering (QELS) revealed that there was no sign of agglomeration and the particles were spherical in shape and well dispersed. A magnified view of a gold nanoparticle is presented in figure 2(c), a typical high-resolution TEM micrography. Figure 2(c) displays fringe patterns, indicating the crystalline nature of these gold nanoparticles.
Figure 2. (a) The quasi-elastic light scattering (QELS) data, (b) TEM micrograph of the gold nanoparticles and (c) magnified TEM micrography revealing fringe patterns and crystalline nature of gold nanoparticles.
Download figure:
Standard image High-resolution image3.1. X-ray diffraction analysis
Gold crystallite sizes were obtained as an average of the three different crystallite size-values estimated from the line width of the (111), (200), (220) diffraction lines by using the Scherrer equation. XRD patterns of the gold nanoparticles prepared are depicted in figure 3 with 2 θ values between 2° and 90°. The XRD pattern of gold nanoparticles in figure 3 shows four characteristic peaks for 2 θ at 38.00°, 44.48°, 64.4° and 77.6° marked indices of (111), (200), (220) and (311) planes, respectively. A significant (111) diffraction peak suggests a prominent growth of network structure along (111) planes compared to (200). Additionally, the other prominent diffraction peak (220) points to the anisotropic (network) nature of the nanoparticles.
Figure 3. XRD of PEI reduced and stabilized gold nanoparticles.
Download figure:
Standard image High-resolution image3.2. Electron diffraction analysis
Figure 4 shows the electron diffraction patterns of the gold nanoparticles. The diffraction pattern was obtained by aligning the electron beam perpendicular to the facet of the nanoplate. The Debye–Scherrer rings originating from the gold FCC structure are clearly observed because of the sloping background and the overlapping of peak tails for the adjacent (111) and (200) peaks and (311) and (222) peaks.
Figure 4. Selected area electron diffraction (SAED) of PEI reduced and stabilized gold nanoparticles.
Download figure:
Standard image High-resolution image3.3. Energy dispersive x-ray analysis
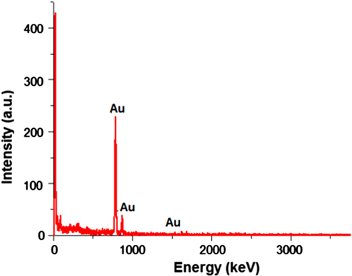
Energy dispersive x-ray (EDX) was performed for synthesized gold nanoparticles. The EDX spectrum given in figure 5 shows gold as the only elementary component.
Figure 5. EDX spectrum of PEI reduced and stabilized gold nanoparticles.
Download figure:
Standard image High-resolution image3.4. Zeta potential of gold nanoparticles
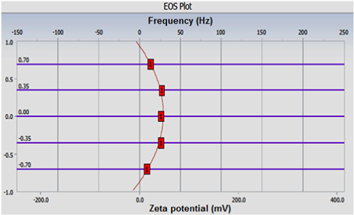
Zeta potential of the nanoparticles was measured using the Delsa™Nano C (size and zeta potential analysis with a duo laser light). The software calculated zeta potential using phase analysis light scattering. The zeta potential of a nanoparticle refers to the electrostatic potential created as a result of the accumulation of electrons at its surface. The software program calculates zeta potential using the electrophoretic mobility of the sample. The charge and intensity of the electrophoretic mobility and zeta potential are reflective of the charge and intensity of the outermost layer of the nanoparticle. Figures 6 and 7 showed the electro-osmosis (EOS) plot and mobility distribution plot for the gold nanoparticles. It shows that the PEI stabilized gold nanoparticles are positively charged with Zeta potential of +23.45 mV with a mobility value of 1.829e-004 cm2 Vs−1. The conductivity measurement of the particles comes out to be 0.5968 mS cm−1. The high cationic charge density synthesized gold nanoparticles kept each nanoparticle apart which resulted in the formation of stable colloids.
Figure 6. Electro-osmosis (EOS) plot of gold nanoparticles.
Download figure:
Standard image High-resolution imageFigure 7. Mobility distribution plot of gold nanoparticles.
Download figure:
Standard image High-resolution image3.5. Effect of concentration of PEI on size of gold nanoparticles
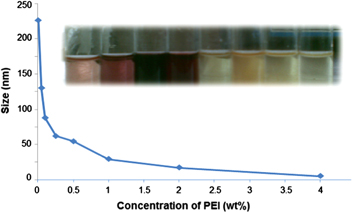
Concentration of PEI plays a major role in deciding the size of the nanoparticles. On varying the PEI concentration, it was observed that the size of the particles decreased with the increase in the concentration of the PEI (table 1 and figure 8). However, when the concentration of PEI was increased beyond 4 wt%, no further decrease in the size of the particles occurred.
Table 1. Variation of concentration of PEI.
| Sample No. | PEI (% w/v) | HAuCl4 (% w/v) | Size (nm) | Polydispersity | Remarks |
|---|---|---|---|---|---|
| a | 4.000 | 1 | 05.10 | 0.616 | Stable |
| b | 2.000 | 1 | 17.10 | 0.579 | Stable |
| c | 1.000 | 1 | 29.15 | 0.300 | Stable |
| d | 0.500 | 1 | 54.16 | 0.412 | Stable |
| e | 0.250 | 1 | 61.65 | 0.313 | Stable |
| f | 0.100 | 1 | 87.54 | 0.506 | Precipited after 12 h |
| g | 0.050 | 1 | 129.9 | 0.282 | Precipited after 6 h |
| h | 0.001 | 1 | 226.0 | 0.293 | Precipited after 2 h |
Conditions: Stock = 3 ml dd-H2O; PEI = 100 μl; HAuCl4 = 100 μl.
Figure 8. Size of gold nanoparticles versus PEI concentration.
Download figure:
Standard image High-resolution imageWhen the concentration ratio of PEI to gold was large enough, the reduction rate of gold chloride was much faster than that of the nucleation rate and almost all the gold ions were reduced to atoms before the formation of proper nuclei. Low concentration of added PEI causes particle aggregation because of the electrostatic attraction of partially coated and uncoated colloids.
3.6. Effect of concentration of gold salt on size of gold nanoparticles
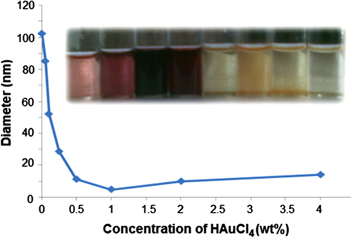
The concentration of the gold salt also plays a crucial role in controlling the diameter of colloidal gold in universal solvent, namely water. On varying the concentration of gold salt keeping the PEI concentration constant, it was observed that at very low concentration of the aqueous gold salt concentration (0.001% w/v), the size of the nanoparticles appeared to be large which finally resulted in the complete precipitation of the nanoparticles (table 2). But, with a gradual increase in the gold salt concentration, the size of the particles decreased. The minimum size of the nanoparticles could be achieved at 1% concentration of the gold salt (table 2 and figure 9). Further increase in the salt concentration resulted in a sudden increase in the particle size.
Table 2. Variation of concentration of HAuCl4.
| Sample No. | PEI (% w/v) | HAuCl4 (% w/v) | Size (nm) | Polydispersity | Remarks |
|---|---|---|---|---|---|
| a | 4 | 0.001 | 102.300 | 0.310 | Precipited in 5 h |
| b | 4 | 0.050 | 085.040 | 0.504 | Precipited in 5 h |
| c | 4 | 0.100 | 051.880 | 0.110 | stable |
| d | 4 | 0.250 | 028.510 | 0.851 | stable |
| e | 4 | 0.500 | 011.060 | 0.011 | stable |
| f | 4 | 1.000 | 004.700 | 0.414 | stable |
| g | 4 | 2.000 | 009.786 | 0.142 | stable |
| h | 4 | 4.000 | 013.892 | 0.350 | stable |
Conditions: Stock = 3 ml dd- H2O, PEI = 100 μl, HAuCl4 = 100 μl.
Figure 9. Diameter of gold nanoparticles versus concentration of HAuCl4.
Download figure:
Standard image High-resolution imageThe above observations may be explained by stating that at low concentration of the salt there will be much less interaction between salt and polymer. Therefore, most of the reaction occurred in the bulk phase and the few particles that formed were not stabilized by the system, resulting in the complete precipitation. However, when the concentration of the salt was increased the interaction between polymer and salt increased and because of the presence of more salt molecule in the solution, they formed closed packed structures. During the reaction, the polymer molecules behaved like stabilizing agents and tightly anchored the particles preventing precipitation. The maximum stability and small particle size was obtained when the concentration of the salt became 1/4 of the PEI concentration (table 2). When salt concentration was increased further the number of salt molecules per unit volume of the solution increased and the effective interaction between the polymers and salt molecules decreased, resulting in the formation of large nanoparticles. Based on the above observations, the salt concentration was selected as 1% w/v for further reactions.
4. Conclusion
We report the first environmentally benign synthesis of positively charged gold nanoparticles. This is a simple polyethlyneimine based synthesis and stabilizing approach for the facile formation of well dispersed, stable, pure, positively charged gold nanoparticles in aqueous solution. The results have implications for the synthesis of biocompatible and sensing materials for biomedical and chemical catalysis applications.