Abstract
TiO2 film photoanodes with a size of 1 × 1 cm2 were fabricated by a spin coating method. Cu-doped TiO2 powder with various Cu concentrations (0.2, 0.4, 0.6 and 0.8 at%) and surfactant were used as starting materials in coating Cu-doped TiO2 thin films onto FTO/glass substrate. Crystalline structure of TiO2 material, microstructure of the photoanode films and their thickness were identified by x-ray diffraction and Raman scattering. Hydrogen generation from water by photoelectrochemical effect in the visible light was observed by recording I/V characteristics of the photoanode in dark and light regimes. The obtained results have shown that the hydrogen generation efficiency of photoanode nonlinearly depends on Cu concentration. The nonlinear dependence of the hydrogen generation efficiency may be due to a change of resistivity of the film photoanode that is related with the random distribution of the hetero-junction between interfaces of TiO2 and CuO nanoparticles.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since their discovery by Honda et al in photoelectrochemical water splitting by using TiO2 photoanode [1] many studies focusing on fabrication of the TiO2 photoanodes active in the visible light were performed [2–4]. Anatase TiO2 is the most interested one, because this phase material is chemically inert, non-toxic and more active in photocatalysis than other phases of titania. Unfortunately, anatase TiO2 has an energy bandgap of 3.2 eV, therefore, it is active only in violet light of wavelength smaller than 390 nm. We know that violet light contributes only about 4% in energy power of the Sun, so TiO2 anatase is not the prospective photocatalytic material applicable for conversion of the Sun's energy. Van Hieu Nguyen et al [5] have reported a comprehensive review on this subject and we can find out information dealing with the preparation of titania and its application in solar energy conversion using photoelectrochemical effects. With the aim of manufacturing TiO2 effectively working with respect to visible light, many authors have modified TiO2 structure by doping anion and/or cation to get TiO2 having narrower bandgap energy. Among the anion doping, C, N, F, P and S are of most interest. Asahi et al [6] doped N into TiO2 by sputtering the TiO2 target in Ar gas containing 40% N and observed a red shift of absorption of the TiO2 sputtered thin film. It was supposed to be related with the creation of acceptor states above valence band that conducted to an overlap of p states of N and s states of oxygen. Other authors reported quite the same experimental results on TiO2 doped with N [7, 8]. Using natural gas flame Khan et al [9] prepared C-doped TiO2 by pyrolysis flame of Ti sheet in the presence of combustion products (CO2 and H2O steam) and observed a five-fold increase in photocatalytic activity of C-doped titania compared to N-doped. Besides researches focused on the anion doping, the research on doping of transition metals such as Cu, Fe and Pt was attempted to prepare visible light responsive nanoparticles and nanocrystalline films. In recent years, research focused on Cu-doped titania increased because Cu can contribute either as a dopant in titania nanocrystal and/or as an incorporated sensitizer in the form of CuO and Cu2O that are responsive to visible light. However, Cu doping could cause the decrease of the conduction bandgap minimum lower than the hydrogen reduction level so that the activity of photocatalytic water splitting is consequently declined. Recently Sun et al [10] have used various methods to prepare Cu-doped titania samples such as sol–gel (SG), wet impregnation (WI), chemical reduction of Cu salt (CR) and in situ photodeposition (PD). As they reported, the sol–gel method has given the best value of the Brunauer–Emmett–Teller (BET) specific surface area of about 87.3 m2 g−1. Titania, having large BET specific surface area, is an expected material used for photocatalysis.
In this report we present our recent research result on hydrogen generation from water by using film photoanodes manufactured by spin coating a solution containing the Cu-doped TiO2 nano powder synthesized by sol–gel method.
2. Experimental
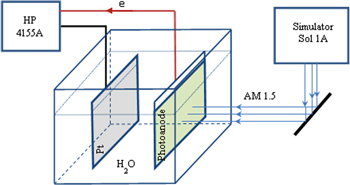
Cu-doped titanium oxide Ti1 − xCuxO2 was manufactured by sol–gel method. Details of the preparation method can be seen in our previous paper [11]. Cu-doped titania photoanode with a size of about 1 cm2 was manufactured by spin coating onto fluorine tin oxide (FTO)/glass substrate of a solution of Cu-doped titania dissolved in a mixture of diethanolamin (C4H11NO2) and ethanol (C2H5OH) with a volume ratio of 1/10. The thickness of Cu-doped titania layer was estimated by Alpha-Step IQ equipment. All the Cu-doped titania films have nearly the same thickness of about 2 micrometer. X-ray diffraction and Raman scattering were used to identify the phase structure of all the samples. The conductivity of all the film samples was estimated by a four probe method. The photocurrent was measured in the dark and light regime by using a HP 4155A semiconductor parameter analyzer and a Sol 1A simulator made in by the US Oriel Company, having power density of 100 mW cm−2 (AM1.5). A schema of photocurrent measurement is presented in figure 1. Using the change of photocurrent measured in dark and light regime, the hydrogen generation efficiency from water was evaluated.
Figure 1. Principle schema of photocurrent measurement used for water splitting evaluation.
Download figure:
Standard image High-resolution image3. Discussion
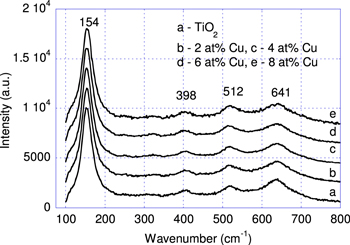
The Cu-doped TiO2nano powder samples were synthesized with Cu concentration changed in a range of 0.0–0.8 at%. Figure 2 presents x-ray patterns of all the Ti1 − xCux O2 samples. It shows that all the samples are considered being pure TiO2 anatase phase. However, there is a very weak x-ray peak marked with (*) sign at 2θ of 31° that is identified to the brookite phase. Figure 3 presents Raman spectra of the Cu-doped titania samples. One can clearly observe a strong vibrational mode at 152 cm−1, and three weak vibrational modes at 398 cm−1, 512 cm−1 and 641 cm−1. These Raman modes reconfirm again that the samples are of pure anatase phase of TiO2. Based on the obtained x-ray patterns the grain size of the Ti1 − xCux O2 nanopowder was estimated. It has been shown that the grain size of the Ti1 − xCux O2 nanoparticles is about 10 nm (this can be seen in figure 2). The estimated active surface area is very high (85 cm2 g−1), comparable to that reported by Sun et al [10].
Figure 2. X-ray patterns of the TiO2-doped with various Cu concentrations (a) 0 at%, (b) 2 at%, (c) 4 at%, (d) 6 at% and (e) 8 at%, and annealed at 450 °C.
Download figure:
Standard image High-resolution imageFigure 3. Raman spectra measured at room temperature of the Cu-doped TiO2nano powder samples.
Download figure:
Standard image High-resolution imageThis obtained result suggests a possibility of using Cu-doped titania nanomaterial in photoelectrochemical applications such as DSSC solar cells, water splitting [12, 13]. Using this nano Ti1 − xCux O2 powder we have fabricated TiO2 photoanodes with the structure of glass/FTO/Ti1 − xCux O2 by spin coating technique. Using these photoanodes we have measured the photocurrents in the dark and light regimes by using a measurement schema as presented in figure 1. The photocurrents recorded in dark and light regimes of the photoanode samples are presented in figure 4. It shows that the photocurrents measured in the dark and light regimes are different and depend on Cu-doped concentration. In principle, the difference of photocurrents measured in dark and light regimes is the response of the photoanode to sun light. Therefore, it is necessary to describe the difference of photocurrents in demand to evaluate the hydrogen generation efficiency for all the photoanodes. From the photocurrent curves presented in figure 4 the difference Δjsc of the photocurrent at zero applied voltage was described for all the photoanodes and the given result has been presented in figure 5. It shows that the difference of photocurrent increases with Cu concentration and reaches maximal value at a concentration of 4 at%, after that it decreases with increasing Cu concentration.
Figure 4. Photocurrent of the Ti1 − xCux O2 photoanode illuminated by the AM1.5 sunlight, (a)—x = 0.02, (b)—x = 0.04, (c)—x = 0.06, and (d)—x = 0.08.
Download figure:
Standard image High-resolution imageFigure 5. The difference of photocurrents measured in dark and light regimes.
Download figure:
Standard image High-resolution imageBased on the result presented in figure 5 we attempt to evaluate hydrogen generation efficiency for all the photoanodes in dependence of the Cu concentration. First, we would like to state important information that the photocurrent does not depend on distance between the working photoanode and counting Pt electrode. It means that the photocurrent depends only on the generation and separation of charge in the photoanode and the transport of the generated electron and hole to surface of the electrodes to take oxidation and reduction of H+ and O2− in water. This fact suggests that the ions H+ and O2− are waiting for reaction with electron and hole at surface of electrodes.
The efficiency of hydrogen generation can be estimated by the following equation
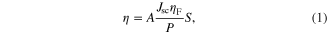
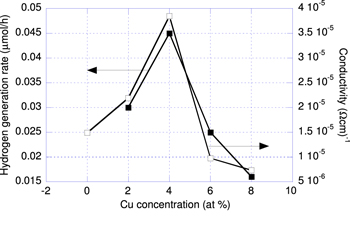
where A is constant 1.23 V, Jsc(mA/cm2) is shortcut photocurrent estimated at zero applied voltage, ηF is Faraday efficiency, S(cm2) is illuminated photoanode area, P(mW) is light power. In our case S and P are 1 cm2 and 100 mW cm−2, respectively. For estimating the hydrogen generation efficiency we use the given different photocurrent measured in the dark and light regime that is presented in figure 5. Using equation (1) and data given from figure 5, we calculated hydrogen reduction efficiency of the photoanodes. Besides, we can calculate the amount of hydrogen generated rate in one hour from the different photocurrent ΔJsc. Since the hydrogen generation and hydrogen generated rate in one hour are linearly dependent quantities, for the convenience we draw and use the curve of hydrogen generated rate in one hour versus Cu concentration as presented in figure 6.
Figure 6. Hydrogen generated in 1 h and conductivity of the Cu-doped TiO2 photoanode versus Cu concentration.
Download figure:
Standard image High-resolution imageConsequently, the hydrogen generation rate has the same dependence on Cu concentration as the difference of the photocurrent presented in figure 5. It is very interesting that the conductivity and hydrogen generated rate of the TiO2 photoanode doped with Cu simultaneously have maximal values at a concentration of 4 at%. With the aim of explaining this coincidence, we have to search absorption spectra of the film photoanodes as presented in figure 7. It is clear that the absorption edge of all the photoanodes has shifted to the longer wavelength. This shift slightly depends on Cu concentration. The shift of the absorption edge is evidence to suggest that the number of Cu substituted for Ti increases as Cu dopant concentration increases. As is shown in [5], a substitution with high Cu concentration may cause a lowering of the conduction band minimum below the hydrogen reduction level that could decline the photocatalytic activity of the photoanodes. However, the substitution of Cu for Ti creates impurity states and consequently increases conducting charge that increases conductivity and then consequently increases photocatalytic activity of the photoanodes. This means that there are at least two competitive processes affecting the photocatalytic activity of photoanodes.
Figure 7. Absorption spectra of the Cu-doped TiO2 film photoanodes.
Download figure:
Standard image High-resolution imageWe would like to emphasize here that the substitution of Cu increases charge concentration in photoanode material that should increase its conductivity since Cu doping concentration increases. On the contrary, the conductivity of the photoanodes really decreased when Cu concentration increased higher than 4 at%. So we have to suggest two other reasons to cause the decrease of the conductivity of the photoanodes when Cu concentration is higher than 4 at%. The first reason is related with the decrease of the charge mobility. The second is related with the random arrangement of two type semiconductor nanoparticles in the photoanodes. It is clear to see that in the absorption spectra there exists a flat absorption tail in the wavelength range larger than 500 nm for the photoanodes with Cu concentration larger than 2 at%. This absorption tail intensity increases with increasing Cu concentration. We consider that the tail is related with absorption band of copper oxide located at TiO2 grain surface. The given absorption spectra are evidence to confirm that the amount of copper oxide located on grain surface of TiO2 nanoparticle increases with increasing Cu concentration. In principle, TiO2 and CuO are incorporated together as two n-type and p-type semiconductors having different bandgap. Up to now this type of incorporation is believed to improve co-photocatalytic activity. Conversely, we received the opposite result in our case; the hydrogen generation efficiency decreases with increasing Cu concentration higher than 4 at%. As we have known, copper oxide is a p-type semiconductor, then it has created p/n hetero-junctions working as a diode that randomly connected. When the CuO amount at TiO2 grain surface increases, the pair number of p/n hetero-junctions connected in the opposite direction increase, so that conductivity of the photoanodes decreases. This is a main reason to reduce charge transfer in photoanodes and consequently to decrease the photocatalytic activity.
4. Conclusion
Cu-doped TiO2 photoanodes were manufactured by spin coating method. The Cu substitution for Ti abnormally affects conductivity of the photoanode material. It is supposed to be related mainly with the increase in the amount of CuO at grain surface of TiO2 nanoparticles that reduces conductivity of the photoanode material due to the increase of the pair number of p/n hetero-junction connected in the opposite direction. Consequently, the hydrogen reduction efficiency also decreases due to the reduction of charge transfer in the photoanode. This obtained result suggests that the incorporation of two semiconductors is not the appropriated method to design photoanodes used for phoelectrochemical applications.
Acknowledgement
This investigation was completed with a financial support of the VAST for the research project under Grant number of VAST03.08/12-13. The authors are grateful to Academician Nguyen Van Hieu for his support in opening the new promising research subject as well as in successful scientific suggestions and discussions. The Key Laboratory for the Electronic Materials and Devices of Institute of Materials Science are also acknowledged.