Abstract
The aim of this study is to fabricate a nanoparticle formulation of curcumin using a relatively new vehicle as the matrix polymer: poly(lactic-co-glycolic acid) (PLGA)- polyethylene glycol (PEG) diblock copolymer, and to investigate the effects of the various processing parameters on the characteristics of nanoparticles (NPs). We successfully synthesized the matrix polymer of PLGA-PEG by conjugation of PLGA copolymer with a carboxylate end group to a heterobifunctional amine-PEG-methoxy using N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide as conjugation crosslinkers. The composition of the formed product (PLGA-PEG) was characterized with 500 MHz 1H nuclear magnetic resonance (NMR). The conjugation of PLGA-PEG was confirmed using Fourier transform infrared (FTIR) spectrum study. This diblock copolymer was then used to prepare the curcumin-loaded NPs through nanoprecipitation technique. With this method, we found that the size distribution depends on the type of solvent, the concentration of polymer and the concentration of surfactant. The particle size and size distribution were measured by dynamic light scattering (DLS). Transmission electron microscope (TEM) and scanning electron microscope (SEM) were used to confirm the size, structure and morphology of the successfully prepared NPs. All of our results showed that they are spherical and quite homologous with mean diameter around of 100–300 nm. Further, we evaluated encapsulation efficiency and some characteristics of NPs through high performance liquid chromatography (HPLC) analyses, zeta-potential measurements and x-ray diffraction studies. The HPLC analyses were performed to determine the amount of curcumin entrapped in NPs. The zeta-potential measurements confirmed the stability of NPs and the successful encapsulation of curcumin within NPs and the x-ray diffraction patterns showed the disordered-crystalline phase of curcumin inside the polymeric matrix.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Curcumin (Cur) is chemically a yellow polyphenol, diferuloylmethane extracted from the rhizomes of turmeric (curcuma longa). It possesses low intrinsic toxicity along with a wide range of pharmacological activities that include antitumor, anti-amyloid, antioxidant and anti-inflammatory properties [1–10]. Preclinical studies of Cur have shown its ability to inhibit carcinogenesis in a variety of cell lines that include breast, cervical, colon, gastric, hepatic, leukemia, oral epithelial, ovarian, pancreatic and prostate cancer [2–5]. The ability of Cur to induce apoptosis in cancer cells without cytotoxic effects on healthy cells makes it a potential compound for drug development against cancer [11]. Despite all these promising characteristics, a major problem with Cur is its extremely low solubility in aqueous solution (2.99 × 10−8 M) and its poor bioavailability, which limits its clinical efficacy [12]. Indeed, studies over the past three decades related to absorption, distribution, metabolism and excretion of Cur have revealed poor absorption and rapid metabolism of Cur that severely curtails its bioavailability [13–15].
In order to overcome these drawbacks, some of the adjuvants which can block metabolic pathway of Cur are one of the major means that are being used to improve its bioavailability [11, 16]. Delivery of Cur through encapsulation in polymeric micelles, liposomes and lipid-based nanoparticles (NPs) are other promising novel formulations which appear to provide longer circulation, better permeability and resistance to metabolic processes. In this study, we have developed Cur nanoparticulate formulation with a diblocked copolymer. Reported and used as a drug delivery vehicle in recent years, the block copolymers which consist of hydrophilic and hydrophobic segments, have attracted significant attention for their use with many different hydrophobic drugs because of their ability to form NPs self-assembly in aqueous solutions. These NPs provide a core–shell structure in which the drug in the core is surrounded by a hydrophilic outer shell that permits prolonged circulation in the blood and reduces uptake by the liver and spleen [17, 18].
In this report, the poly(lactic-co-glycolic acid) (PLGA) diblocked with polyethylene glycol (PEG) was synthesized and its structure proven with proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared (FTIR) studies. Cur was then entrapped in this obtained carrier material using nanoprecipitation technique. The size of Cur-loaded PLGA-PEG NPs was controlled by changing solvent, polymer concentration and surfactant concentration. The studies of size distribution, morphology and structure of Cur-encapsulated PLGA-PEG NPs were investigated by dynamic light scattering (DLS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The Cur entrapment ability of PLGA-PEG NPs was evaluated using HPLC. Moreover, the other physicochemical characteristics of these NPs were also conducted.
2. Materials and methods
2.1. Materials
Curcumin (⩾94% curcuminoid content) was purchased from Sigma Aldrich (USA) and used without further purification. Poly(lactic-co-glycolic acid) chosen in this study is US food and drug administration (FDA) approved polymer and has been used for oral formulation [17]. This material is a copolymer of polylactic acid and polyglycolic acid. We obtained PLGA with a 50:50 monomer ratio, carboxylate terminated, and inherent viscosity of 0.18 dl g−1 from LACTEL absorbable polymers (USA) and used it as-received. Methoxy PEG amine (m-PEG-NH2), a non-toxic, water-soluble polymer with proven biocompatibility was from JENKEM (USA).
All of the solvents used: dichloromethane, ethyl ether, methanol, acetonitrile and acetone were purchased from Merck (Germany).
All other chemical reagents: N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), polyvinyl alcohol (PVA) (Mw = 9000–10 000) were procured from Sigma Aldrich (USA).
2.2. Synthesis of PLGA-PEG diblock copolymer
The conjugation of PLGA with a carboxylate end group to a heterobifunctional amine-PEG-methoxy was carried out in two steps by using EDC and NHS as conjugation crosslinkers. Briefly, PLGA was dissolved in dichloromethane (DCM) and stirred at room temperature for 4 h in the presence of EDC and NHS (1:5 molar ratio of PLGA:EDC and 1:5 molar ratio of PLGA:NHS) to form the PLGA-NHS. This ester was precipitated with ice-cold ethyl ether and then washed three times with an ice-cold mixture of ethyl ether/methanol to remove any NHS and EDC residuals. Any traces of the washing solution were removed under vacuum for 2 h. The obtained product was re-dissolved in DCM. This solution was incubated in the presence of m-PEG-NH2 (1:1.5 molar ratio) for 24 h under gentle stirring. The reaction product PLGA-PEG was precipitated out by adding the ice-cold methanol and repeatedly washed to remove all excess unreacted PEG. The final PLGA-PEG product was vacuum-dried for 5 h and stored at 4 °C until use.
2.3. Copolymer characterization: 1H NMR and FTIR spectra studies
The proton NMR spectrum has been used for structure analysis. Spectra were recorded on a Bruker AV 500 MHz spectrometer. Deteurated chloroform (CDCl3) was used as solvent. The standard 1H NMR spectra were analysed to verify m-PEG-NH2 conjugation to PLGA.
Conjugation of PLGA-PEG is also confirmed using FTIR spectra study. Measurement was carried out on TENSOR™37-BRUNER spectrometer. The product PLGA-PEG was crushed with KBr to get the pellet by applying a pressure of 300 kg cm−2. FTIR spectrum was obtained in the range of 400–4000 cm−1.
2.4. Preparation of Cur-encapsulated PLGA-PEG NPs
Cur was encapsulated in NPs with PLGA core covered with PEG at the surface. In this study, such NPs were successfully prepared through the nanoprecipitation technique. Briefly, PLGA-PEG and Cur were dissolved in appropriate solvent. Further, this polymer/drug mixture was added drop wise to stirring water containing a surfactant. From the formed NP suspension was removed the remaining organic solvent by vacuum drying and then it was concentrated by cool-centrifugation at 15 000 rpm for 30 min The obtained pellets continued with a free-drying step, whereas the supernatant was collected to quantify the non-encapsulated Cur. The encapsulation efficiency (%) was calculated from this amount of non-encapsulated Cur and the initial amount of Cur used.
We studied the effect of various processing parameters on particle size. The processing parameters include: the type of solvent which dissolves polymer and Cur, the polymer concentration in the organic phase, the surfactant concentration in the aqueous phase and the aqueous phase volume.
2.5. Characterization of NPs
The particle size and size distribution were measured by DLS (LB502, Japan) at 25 °C, scattering angle of 90 °C.
The particle size and morphological examination of the NPs were performed with TEM (JEM 1400, Japan) and SEM (JSM 6480LV-JEOL, Japan) measurements. The samples were placed on carbon-coated copper grids for viewing by TEM. For SEM, the samples from dry powder particles were prepared by gently dipping gold grids into the dry NPs.
Zeta potential is an indicator for charge present on the surface of NPs, which deals with stability of formulations. A Zetasizer was used to measure the zeta potential for the diluted nanoparticulate suspension.
XRD study was done to know the nature of Cur after encapsulation into the polymeric NPs. The patterns of native Cur, PLGA-PEG NPs and Cur-loaded PLGA-PEG NPs were obtained using Bruker D8 diffractometer. The measurements were performed at a voltage of 40 kV and 25 mA. The scanned angle was set from 2° ≦̸ 2θ ⩾ 50° and the scan rate was 2° min−1.
3. Results and discussion
3.1. Synthesis of PLGA-PEG diblock copolymer
PLGA has been among the most attractive polymeric candidates used to fabricate devices for drug delivery and tissue engineering applications [17]. PLGA-PEG is a non-ionic diblock copolymer consisting of hydrophilic PEG and hydrophobic PLGA segment. In this diblock type, PLGA is a hydrophobic core that enables entrapping hydrophobic molecules to protect them from the surrounding environment while PEG chains orient themselves towards the external aqueous phase in micelles, thus surrounding the encapsulated species. This layer of PEG acts as a barrier and reduces the interactions with foreign molecules by steric and hydrated repulsion, giving enhanced shelf stability [17, 18]. In this study, the PEG-PLGA diblock copolymer was synthetized successfully. The route of synthesis is that carboxylates (−COOH) may be reacted to NHS in the presence of a carbodiimide such as EDC, resulting in a semi-stable NHS ester, which may then be reacted with primary amines (−NH2) to form amide crosslinks. In our experiment, bi-functional PEG containing a methoxy group at one terminus and an amino group at the other was used to form methoxy-PEG-PLGA.
One of the important points in the process of synthesis is the control of solvent volume. For maximum conjugation efficiency, we should use an appropriate volume of solvent. A low concentration of reagent reduces the conjugation performance. But too concentrated a solution of reagent is not requested because it becomes more difficult to properly wash the obtained product.
The structure of PLGA, m-PEG-amine and PLGA-PEG were shown in figure 1. Proton NMR revealed characteristic peaks of PLGA at δ(ppm) = 1.5 (−CH3) (α), δ(ppm) = 4.8 (−CH2−) (β) and δ (ppm) = 5.2 ppm (−CH−) (γ) in all PLGA dissolved polymer samples (figure 1(a)). Peaks were observed at δ(ppm) = 3.6 (−CH2) (ε) and δ(ppm) = 2.0 (−NH2) (ζ) in m-PEG-amine corresponding to the proton NMR spectra of m-PEG-amine. In proton NMR of formed product (figure 1(c)), the signal at δ(ppm) = 2.0 was entirely absent, which demonstrated the conjugation of the amine group at the end of m-PEG-amine to the carboxylate group of PLGA.
Figure 1. Proton NMR spectra of (a) PLGA-COOH, (b) m-PEG-NH2, (c) m-PEG-PLGA.
Download figure:
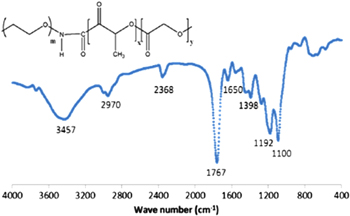
Standard image High-resolution imageFurther to confirm the success of PLGA-PEG conjugation, FTIR analysis was taken in to consideration. Figure 2 shows the FTIR spectrum of our obtained product. In this spectrum, we pointed out two important peaks. The strong peak observed at 1767 cm−1 can be due to C = O double bond and the peak found at 3457 cm−1 can be attributed to N-H streching vibration in our product. These results confirmed that PEG conjugated with PLGA, as a result, PLGA-PEG was produced.
Figure 2. FTIR spectrum of our product PEG-PLGA.
Download figure:
Standard image High-resolution image3.2. Preparation of Cur-encapsulated PLGA-PEG NPs by solvent displacement method
The Cur was successfully incorporated into PLGA-PEG NPs by solvent displacement method (71%). The basic principle of this preparation technique is based on the interfacial deposition of a polymer after displacement of a semi-polar solvent, miscible with water from a lipophilic solution. Rapid diffusion of the solvent into non-solvent phase results in the decrease or interfacial tension between the two phases, which increases the surface area and leads to the formation of small droplets of organic solvent. The previous studies indicated that the process of particle formation in this method comprises three stages: nucleation, growth and aggregation. The rate of each step determines the particle size. The researches performed up to now have focused on the phase mixing method, the organic phase addition rate, the stirring system, the stirring time and the temperature. We did not study here these operating variables. We investigated the effect of the parameters concerned in three basic components of a nanoprecipitation system include: the polymer, the organic solvent and the non-solvent phase.
3.2.1. Influence of type of solvent
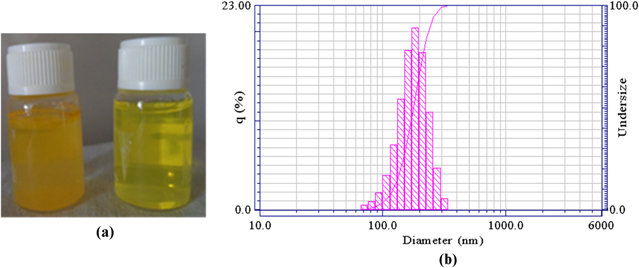
As a starting point for controlling the NP size distribution, we first studied the effect of varying the type of organic solvent used to solubilize the drug and polymer. Previous studies have suggested that the miscibility of the organic solvent in water can impact NP size for a given solvent: water system [19, 20]. We chose to investigate the relationship of NP size and solvent miscibility with water using three organic solvents. As shown in table 1, the sizes of PLGA-PEG NPs and the water-miscibility of the three organic solvents used in this study were generally correlated: an increase of water miscibility led to a decrease in the mean NP size, with all other formulation parameters held constant. The most water miscible solvent tested resulted in the smallest particles which are presumably due to more efficient solvent diffusion and polymer dispersion into water (figure 3).
Table 1. Effect of type of solvent on particle size of Cur-loaded PLGA-PEG NPs prepared by the nanoprecipitation method.
| Run | Solvent | Particle sizea (nm) | Size distributiona (nm) |
|---|---|---|---|
| 1 | Acetone | 80.2 | 33.8–258.6 |
| 2 | Acetonitrile | 137.4 | 50.7–668.7 |
| 3 | Tetrahydrofuran | 121.8 | 44.3–296.2 |
Figure 3. (a) Free Cur in water and Cur-loaded NPs suspension, (b) Hydrodynamic particle diameter of Cur-loaded NPs.
Download figure:
Standard image High-resolution image3.2.2. Influence of polymer concentration in the organic phase
The effect of PLGA-PEG concentration on particle size was studied. The experiments were conducted with four PLGA-PEG concentrations of 5, 10, 15 and 20 mg mL−1 while keeping other processing parameters at standard conditions and the results are shown in table 2 and figure 4.
Table 2. Effect of PLGA-PEG concentration on particle size of Cur-loaded PLGA-PEG NPs prepared by the nanoprecipitation method.
| Run | Polymer concentrationa (mg mL−1) | Particle sizeb (nm) | Size distributionb (nm) |
|---|---|---|---|
| 1 | 5 | 137.4 | 50.7–583.9 |
| 2 | 10.0 | 193.2 | 66.6–445.1 |
| 3 | 15.0 | 222.2 | 87.3–509.8 |
| 4 | 20.0 | 257.4 | 114.5–583.9 |
Figure 4. Effect of polymer concentration on particle size of Cur-loaded NPs prepared by the nanoprecipitation method.
Download figure:
Standard image High-resolution imageThe increase in the polymer concentration leads to a gradual increase in NP diameters. This phenomenon can be explained based on the viscosity of dispersed phase. The increasing polymer concentration usually accompanies the increasing viscosity of dispersed phase. As a result, the droplet is formed bigger leading to bigger NP diameters. The control of NP sizes by changing polymer concentrations has previously been reported in emulsification evaporation method. It was explained that the increase in polymer concentration leads to an increase in the viscous forces resisting droplet breakdown by sonication. Although solvent displacement method is a spontaneous process without additional mechanical energy, polymer chain association could govern nucleation and growth rates. In addition, rapid solvent diffusion towards the aqueous phase could be hindered [21, 22].
3.2.3. Influence of concentration of surfactant in the aqueous phase
In our preparation process, the PVA solution acts as the stabilizer. The addition of surfactants helps to preserve the NP suspensions from agglomeration over long storage periods. Although a surfactant is not required to ensure the formation of particle by solvent displacement method, the particle size is influenced by the surfactant nature and concentration. It was reported that an increase in the amount of PVA in the formulation may lead to even smaller particle size due to the tight surface that was formed from PVA macromolecular chains of high concentration [19, 20]. However, too much PVA is not suggested because the increasing PVA concentration is usually accompanied by the increasing viscosity. In addition, removal of the excess amount of PVA is difficult. Thus, we performed the experiments with four PVA concentrations of 0, 0.5, 0.75 and 1% while keeping other processing parameters at standard conditions. Table 3 illustrates this impact.
Table 3. Effect of surfactant concentration on particle size of Cur-loaded PLGA-PEG NPs prepared by the nanoprecipitation method.
| Run | Surfactant concentration (%) | Particle sizea (nm) | Size distributiona (nm) |
|---|---|---|---|
| 1 | 0 | 258.9 | 100–583.9 |
| 3 | 0.5 | 185.5 | 58.1–388.6 |
| 4 | 0.75 | 157.9 | 50.7–445.1 |
| 5 | 1 | 137.4 | 80.7–583.9 |
3.3. Characterization of NPs
The SEM images (figure 5) confirmed that the produced particles are spherical. The particles are agglomerated, which was certainly caused by the centrifugation process. Figure 6 illustrates TEM images showing the formation of spherical, smooth and nano size particles. The diameter of particles observed in TEM was 100–200 nm, which is slightly smaller than that detected by the DLS method. This result is quite reasonable since particle size determined by DLS represents its hydrodynamic diameter, whereas that obtained by TEM is related to the collapsed NPs after water evaporation.
Figure 5. SEM images of Cur-loaded NPs.
Download figure:
Standard image High-resolution imageFigure 6. TEM images of Cur-loaded NPs.
Download figure:
Standard image High-resolution imageZeta potential is an indicator for charge present on the surface of NPs, which deals with stability of formulations. Our results showed high zeta potential (−32 mV) of NPs. Such higher zeta potential helps the formulations repel each other, which ensures long-term stability and avoids particle aggregation.
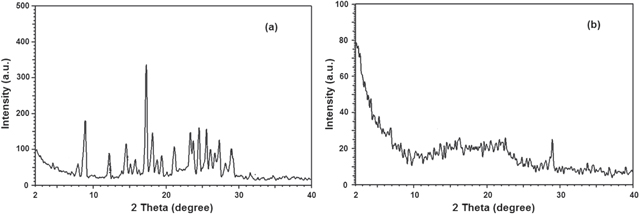
The x-ray diffraction (XRD) study was further carried out to understand the nature of curcumin in our nanoparticle formulation. The XRD patterns of Cur and Cur-loaded PLGA-PEG NPs are presented in figure 7. The characteristic peaks of native Cur exhibited, as shown in figure 7(a), demonstrated the traits of high crystalline structure.
Figure 7. The XRD patterns of (a) Cur and (b) Cur-loaded PLGA-PEG NPs.
Download figure:
Standard image High-resolution imageHowever, there were no characteristic Cur peaks observed when entrapped in NPs (figure 7(b)). This absence of detectable crystalline domains of Cur in nanoparticulate Cur clearly indicates that Cur encapsulated in NPs is in the amorphous or disordered-crystalline phase or in the solid-state solubilized form in the polymer matrix. This disordered-crystalline phase or Cur inside the polymeric matrix helps in sustained release of the drug from the NPs. Presence of drug in crystalline form inside NPs hampers its release as such large sized molecules cannot diffuse from the small pores of the NPs. However, if the drug is amorphous or in dis-ordered crystalline phase, easy diffusion of drug molecules can occur through the polymeric matrix, leading to a sustained release of the encapsulated drug.
4. Conclusion
PLGA diblocked with PEG was synthesized by conjugation crosslinkers using EDC and NHS catalyst. Core–shell type NPs were formulated with this diblock copolymer through solvent displacement method and successfully encapsulated hydrophobic Cur. Nanoprecipitation technique offers the advantages of simple and gentle formulation under ambient conditions without the use of chemical additives or harsh formulation processes. Solvent, concentration of polymer in the organic phase and concentration of surfactant in the aqueous phase were important to obtain small size particles. The Cur-loaded PLGA-PEG NPs developed here which has a small and appropriate size (100–300 nm) and high surface charge. The results implied that PLGA-PEG could be a promising drug carrier for water poorly soluble drug. In future studies, the in vitro and in vivo efficacy of Cur-loaded PLGA-PEG NPs will be evaluated in order to analyze the ability to improve the oral bioavailability of Cur.
Acknowledgments
This research is partial of project 'Fabrication of poly(lactic-co-glycolic acid)—polyethylen glycol nanoparticles to improve the bioavailability of Curcumin'. The authors are grateful to Vietnam National University in Ho Chi Minh City for the financial support.