Abstract
In recent years, nanoscience and nanotechnology have emerged as a new area of fundamental science and are receiving global attention due to their extensive applications. Conventionally nanoparticles were manufactured by physical and chemical techniques. The recent development and implementation of new technologies have led to a new trend, the nano-revolution unfolding the role of plants in bio- and green synthesis of nanoparticles which seems to have drawn a quite unequivocal attention to the synthesis of stable nanoparticles. Although nanoparticles can be synthesized through many conventional methods, biological route of the synthesis is more competent than the physical and chemical techniques. Biologically synthesized nanoparticles have enjoyed an upsurge of applications in various sectors. Hence, the present study envisions biosynthesis of nanoparticles from plants which are emerging as nanofactories. Hence, the present review summarizes the literature reported thus far and envisions plants as emerging sources of nanofactories along with applications, the mechanism behind phytosynthesis of nanoparticles and the mechanism of antibacterial action of nanoparticles.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology provides the ability to engineer the properties of materials by controlling their size, and this has driven research towards a multitude of potential uses for nanomaterials. According to the National Nanotechnology Initiative, USA, nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers [1, 2].
Nanotechnology includes various fields of science such as surface science, organic chemistry, molecular biology, semiconductor physics and micro fabrication [3, 4]. Nanotechnology has the potential to create many new materials and devices with a vast range of applications, such as in medicine, electronics, biomaterials and energy production etc.
Nanoscience is a boon and can bring unanticipated transformation in diverse areas of research and applications. Nanotechnology in recent years has emerged as an enabling technology for the development of computer chips. But in recent years, nanotechnology has also shown its role in a number of fields such as medicine, healthcare, food, agriculture etc. An important area of research in this field is the synthesis of nanoparticles with different chemical compositions, sizes, shapes, and controlled disparities. During the last decade, the biosynthesis of noble metal nanoparticles (silver, gold, platinum, palladium and other nanoparticles as well) has received considerable attention due to the growing need to develop environmentally friendly technologies in material synthesis [5–8].
2. Methods of the synthesis of nanoparticles
Nanoparticles are broadly classified in to two categories, Organic nanoparticles and inorganic nanoparticles. Organic nanoparticles include carbon nanoparticles and inorganic nanoparticles include metal nanoparticles (Ag, Au, Pt, and Pd), magnetic nanoparticles and semi-conductor nanoparticles (TiO2, SiO2, ZnO2).
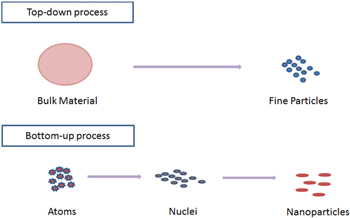
In general there are two processes used in the synthesis of nanoparticles viz. top-down process and bottom-up process. In top-down process bulk material is broken down into particles at nanoscale with different lithographic techniques such as grinding, milling etc, and in bottom-up approach, atoms self-accumulate to new nuclei which convert into a particle of nanoscale (figure 1).
Figure 1. Procedure for the synthesis of nanoparticles.
Download figure:
Standard image High-resolution imageNanoparticles can be produced by either conventional physical and chemical methods or modern green synthesis. The conventional methods include ion sputtering, solvothermal synthesis, reduction and sol–gel technique. However, overall these methods are energy demanding, expensive, and are not eco-friendly. Due to the utilization of toxic chemicals and nonpolar solvents and later on synthetic additives or capping agents, their applications in clinical and biomedical fields are prohibited. Consequently, the need for the development of a clean, reliable, biocompatible, benign, and eco-friendly process to synthesize nanoparticles leads to turning researchers toward 'green' chemistry and bioprocesses [9].
The possibilities of employing plants in the deliberate synthesis of nanoparticles are attracting growing interest as an important source towards a reliable and environmentally benign method of metallic nanoparticles synthesis and its characterization (figure 2).
Figure 2. Steps involved in the synthesis of nanoparticles.
Download figure:
Standard image High-resolution imageThe green methods of synthesizing nanoparticles using naturally occurring reagents such as vitamins, sugars, plant extracts, biodegradable polymers and microorganisms as reductants and capping agents are proven to be more environmental friendly and effective. Plant parts such as leaf, root, latex, seed, and stem are being used for metal nanoparticle synthesis. The key active agents in some of these syntheses are believed to be polyphenols present, for example, in tea, wine and winery waste, red grape pomace. Greener synthesis of nanoparticles provides advancement over other methods as it is simple, cost-effective, and relatively reproducible and often results in more stable materials [10]. Microorganisms can also be utilized to produce nanoparticles but the rate of synthesis is slow and only a limited number of sizes and shapes are amenable compared to routes involving plant based materials. At present, fungi are gaining worldwide popularity as nano-factories for the green synthesis of nanoparticles [11]. Overall, biological materials provide an environmentally friendly or greener chemical method to produce invaluable materials because the biomaterial based routes eliminate the need to use harsh or toxic chemicals [12]. Mechanisms of these bio-reductive transformations [13–15], as well as catalytic properties of materials obtained via these routes [16–19] have been discussed in a series of reports.
In comparison to microorganisms, the synthesis of nanoparticles by using plant extract (phytosynthesis) is less complex and a single-step process unlike microbial isolation, culturing, maintenance etc, and is also a very rapid and cost-effective approach that can be easily scaled up for bulk production of nanoparticles [20]. Moreover, it has been shown that the rate of nanoparticle synthesis is faster using plants than microbes, and the produced nanoparticles are more stable [21]. In addition, phytosynthesis is truly a 'green' synthesis route in comparison to other known methods of nanoparticle synthesis. Plants are known to harbor a broad range of metabolites. However, their potential is yet to be fully utilized at full throttle for synthesizing metallic nanoparticles. By using plant tissue culture techniques and optimizing the downstream processing, it is possible to synthesize metal nanoparticles at an industrial scale [22]. The aim of this review is to provide the recent trends involved in phytosynthesis of noble metal nanoparticles.
2.1. Biosynthesis of noble metal nanoparticles by plants
Plants system has been evaluated by means of the reliable and easy procedure for the biosynthesis of nanoparticles due to its eco-friendly nature [23, 24]. It has been proved that plants are very suitable for the bio-fabrication of noble metallic nanoparticles.
The recent reports on phytosynthesis of noble metal nanoparticles have been summarized in tables 1, 2 and 3 are discussed below.
Table 1. Silver nanoparticles synthesized by different plant species with their size and shape.
| S.N. | Plant | Plant part | Type | Size | Shape | Reference |
|---|---|---|---|---|---|---|
| 1 | Foeniculum vulgare | Leaf | Ag | 37.25 nm | spherical | [25] |
| 2 | Turnera ulmifolia Linn | Leaf | Ag | 10–17 nm | multiple | [26] |
| 3 | Solanum torvum | Leaves | Ag | 14 nm | spherical | [27] |
| 4 | Pelargonium graveolens | Leaves | Ag | 16–40 nm | crystalline | [28] |
| 5 | Cochlospermum religiosum | Leaf | Ag | 31 nm | spherical | [29] |
| 6 | Polyalthia longifolia | Leaves | Ag | 25 nm | spherical | [30] |
| 7 | Calotropis gigantean | Leaves | Ag | 83.7 nm, 5.9 nm, 11.8 nm | spherical | [31] |
| 8 | Piper nigrum | Seeds | Ag | 20–50 nm | spherical | [32] |
| 9 | Cassia auriculata | Leaves | Ag | 20 nm | spherical | [33] |
| 10 | Zingiber officinale | Root | Ag | 26 nm | spherical | [34] |
| 11 | Ocimum tenuiflorum | Leaves | Ag | 27 nm | spherical | [35] |
| 12 | Vitex negundo L. | Leaves | Ag | 18.2 nm | spherical | [36] |
| 13 | Elettaria cardamomom | Seeds | Ag | 40–70 nm | spherical | [37] |
| 14 | Morus nigra | Leaves | Ag | 23 nm | cubical | [38] |
| 15 | Polyalthia longifolia | Leaves | Ag | 35 nm | spherical | [39] |
| 16 | Juglans regia L. | Leaves | Ag | 10–50 nm | different shapes | [40] |
| 17 | Hydrilla verticilata | Leaves | Ag | 65 nm | spherical | [41] |
| 18 | Riccia | Leaves | Ag | 20–50 nm | cuboidal and triangular | [42] |
| 19 | Anthoceros | Whole plant | Ag | 20–50 nm | cuboidal and triangular | [43] |
| 20 | Andrographis paniculata Nees | Leaves | Ag | 35–55 nm | spherical | [44] |
| 21 | Punica granatum | Fruit | Ag | 30–40 nm | spherical | [45] |
| 22 | Boswellia ovalifoliolata | Stem Bark | Ag | 30–40 nm | spherical | [46] |
| 23 | Cymbopogan citratus | Leaves | Ag | 32 nm | spherical | [47] |
| 24 | Ocimum sanctum | Leaves | Ag | 18 nm | spherical | [48] |
| 25 | Dillenia indica | Fruit | Ag | 40–60 nm | colloidal | [49] |
| 26 | Arbutus unedo | Leaves | Ag | 20 nm | spherical | [50] |
| 27 | Rosa rugosa | Leaves | Ag | 11 nm and 14 nm | spherical | [51] |
| 28 | Hevea brasiliensis | Latex | Ag | 2–100 nm | spherical | [52] |
| 29 | Emblica officinalis | Fruit | Ag | 10–20 nm | multiple | [53] |
| 30 | Helianthus annus | Leaves | Ag | 19 nm | multiple | [54] |
| 31 | Oryza sativa | Leaves | Ag | 11–25 nm | multiple | [54] |
| 32 | Saccharum officinarum | Leaves | Ag | 20 nm | multiple | [54] |
| 33 | Mimusops elengi Linn. | Leaves | Ag | 55–80 nm | spherical | [55] |
| 34 | Jatropha curcas | Latex, seed | Ag | 10–50 nm | spherical, irregular | [56] [57] |
| 35 | Macrotyloma uniflorum | Seeds | Ag | 12 nm | spherical | [58] |
| 36 | Euphorbia milii | Latex | Ag | 10–50 nm | spherical | [59] |
| 37 | Ixora coccinea | Leaves | Ag | 13–57 nm | spherical | [60] |
| 38 | Terminalia chebula | Fruit | Ag | 25 nm | spherical | [61] |
| 39 | Malva parviflora | Leaves | Ag | 19–25 nm | spherical | [62] |
| 40 | Trachyspermum ammi and Papaver somniferum | Seeds | Ag | 87 and 76 nm | triangle and spherical | [63] |
| 41 | Pithecellobium dulce | Leaves | Ag | 62 nm | spherical rods | [64] |
| 42 | Sesbania grandiflora | Leaves | Ag | 10–25 nm | spherical | [65] |
| 43 | Pinus densiflora | Leaves | Ag | 15–50 nm | cubic | [66] |
| 44 | Ginko biloba | Leaves | Ag | 15–50 nm | cubic | [66] |
| 45 | Azadirachta indica | Leaves | Ag | 10–37 nm | spherical | [67] |
| 46 | Allium sativum | Root | Ag | 7.3 nm | spherical | [68] |
| 47 | Piper betle | Leaves | Ag | 3–37 nm | spherical | [69] |
| 48 | Portulaca oleracea L. | Roots, leaves and stems | Ag | 146 nm, 136 nm and 175 nm | multiple shapes | [70] |
Table 2. Gold nanoparticles synthesized by different plant species with their size and shape.
| S.N. | Plant | Plant part | Type | Size | Shape | Reference |
|---|---|---|---|---|---|---|
| 1 | Rosa rugosa | Leaves | Au | 11–14 nm | spherical | [51] |
| 2 | Emblica officinalis | Fruit | Au | 15–25 nm | multiple | [53] |
| 3 | Aloe vera | Leaves | Au | 15–50 nm | crystalline | [71] |
| 4 | Punica granatum | Seeds | Au | 51.43 nm | spherical | [72] |
| 5 | Menta piperita | Leaves | Au | 15 nm | spherical | [73] |
| 6 | Trigonella foenum-graecum | Seeds | Au | 15–25 nm | spherical | [74] |
| 7 | Cyymbopogon flexuosus | Leaf | Au | 12–30 nm | triangular | [75] |
| 8 | Pelargonium graveolens | Leaves | Au | 20–40 nm | icosahedral | [76] |
| 9 | Tanacetum vulgare | Fruit | Au | 11 nm | triangular | [77] |
| 10 | Eucalyptus camaldulensis, Pelargonium roseum, Azadirachta indica, | Leaves | Au | 5.5–7.5 nm | crystalline | [78] |
| 11 | Magnolia kobus, Diopyrus kaki | Leaves | Au | 5–50 nm | multiple shape | [79] |
| 12 | Coriandum sativum | Leaves | Au | 6.7–57.9 nm | multiple shape | [80] |
| 13 | Mangifera indica | Leaves | Au | 17–20 nm | spherical | [81] |
| 14 | Memecylon edule | Leaves | Au | 10–45 nm | circular, triangular, hexagonal | [82] |
| 15 | Murraya keenigii | Leaves | Au | 20 nm | triangular | [83] |
| 16 | Zingiber officinale | Roots | Au | 5–15 nm | spherical | [84] |
| 17 | Cicer arietinum | Bean, leaf | Au | 10–23 nm | triangular | [85] [86] |
| 18 | Euphorbia hirta L. | Leaves | Au | 6–71 nm | spherical | [87] |
| 19 | Rosa hybrid | Petal | Au | 10 nm | spherical, triangular and hexagonal | [88] |
| 20 | Angelica, Hypericum and Hamamelis | Plant extract | Au | 4–8 nm | spherical, ovals, polyhedral | [89] |
Table 3. Different nanoparticles synthesized by different plant species with their size and shape.
| S.N. | Plant | Plant part | Type | Size | Shape | Reference |
|---|---|---|---|---|---|---|
| 1 | Gardenia jasminoides | Leaves | Pd | 3–5 nm | [90] | |
| 2 | Pinus resinosa | Bark | Pd | 16–20 nm | spherical | [91] |
| 3 | Cinnamom zeylanicum | Bark | Pd | 15–20 nm | crystalline | [92] |
| 4 | Curcuma longa | Tuber | Pd | 10–15 nm | spherical | [93] |
| 5 | Musa paradisica | Peeled banana | Pd | 50 nm | crystalline irregular | [94] |
| 6 | Cinnamomum camphora | Leaves | Pd | 3.2–6 nm | multiple | [95] |
| 7 | Glycine max | Leaves | Pd | 15 nm | spherical | [96] |
| 8 | Doipyros kaki | Leaves | Pt | 2–12 nm | crystalline | [97] |
| 9 | Pinus resinosa | Bark | Pt | 6–8 nm | irregular | [91] |
| 10 | Ocimun sanctum | Leaves | Pt | 23 nm | irregular | [98] |
| 11 | Camellia sinensis | Leaves | Fe | 60 nm | multiple | [99] |
| 12 | Glycine max | Sprout | Fe | 25 nm | irregular | [100] |
| 13 | Hordeum vulgare | Leaves | Fe | 30 nm | irregular | [101] |
| 14 | Rumex acetosa | Leaves | Fe | 10–40 nm | irregular | [102] |
In addition, extracts of several plants (leaf, seed, fruits etc), viz. Argimona maxicana, chick pea, lemongrass, zinger, black pepper, neem, mango etc have been reported to demonstrated their potential in reducing Au(III) ions into gold nanoparticles as well as converting silver ions into silver nanoparticle [20, 32, 75, 76, 81, 84, 103]. Similarly, synthesis of iron, copper, platinum and palladium nanoparticles have been reported using extracts of various parts of different plant species viz., Rumex acetosa, Glycine max, Ocimun sanctum etc [96, 98, 102].
2.1.1. Nano silver
One of the most valuable and formulated metals is silver (nano silver). Due to its antimicrobial properties towards medical and industrial benefits, silver has also been incorporated in filters to purify drinking water and clean swimming pool water (Agency for Toxic Substances and Diseases Registry-ATSDR, 1990 cited in WHO, 2002). A large number of reports have been dedicated to greener synthesis of silver nanoparticles. Nano-silver particles are mostly smaller than 100 nm and consist of about 20–15 000 silver atoms. Singh et al [103] reported the synthesis of highly stable and crystalline silver nanoparticles (15–30 nm) by exposing the aqueous Argimone maxicana leaf extract to silver nitrate solution. The rate of synthesis of nanoparticles was found to be very high when the reaction was started within 5 min (complete reaction time 60 min). This entails that the use of plants instead of microorganisms for biosynthesis of metal nanoparticles is a more rapid and reproducible process.
2.1.2. Nano gold
Gold nanoparticles are the most attractive member of metallic nanoparticles due to their huge applications in fields such as catalysis, nonlinear optics, nanoelectronics, gene expression, and disease diagnosis [78]. Increased environmental concerns over chemical synthesis routes have drawn considerable interest toward phytosynthesis of gold nanoparticles. Shankar et al [75] reported the formation of gold nano-triangles employing the extract of Cymbopogon flexuosus. Similarly, other workers avowed the synthesis of gold nanoparticles using different plants such as Pelargonium graveolens, Zingiber officinale, Rosa hybrid, Chick pea [76, 84, 87, 88].
2.1.3. Other nano metals
Other metallic nanoparticles like Pt, Pd, Cu, Fe have been synthesized by using different plant parts. Palladium nanoparticles synthesized by leaf of Gardenia jasminoides plant [90], bark of Pinus resinosa and Cinnamom zeylanicum plants [91, 92]. These Pd nanoparticles have different sizes and shapes. Pd nanoparticles have catalytic properties and affinity for hydrogen. Similarly, platinum nanoparticles have also been synthesized by using different plants such as Pinus resinosa, Doipyros kaki, Ocimun sanctum [91, 97, 98]. Iron and iron oxide nanoparticles have also been synthesized. Camellia sinensis, Hordeum vulgare, Rumex acetosa plants extracts have been used to produce iron nanoparticles [100–102].
2.2. Mechanism behind phytosynthesis of nanoparticles
Several studies have been initiated on the broadcasting and identification of plants for organized and controlled production of metallic nanoparticles, but very little work has been done to recognize the real mechanism behind the synthesis of nanoparticles [34, 48, 52].
Investigation on the fundamental molecular mechanism is needed to regulate the size and shape of metal nanoparticles. In recent years, numerous theories have been suggested for synthesis of nanoparticles [104–108]. However, the exact mechanism of the phytosynthesis of metal nanoparticles is not yet identified and more revisions are needed [135].
A research group at National Chemical Laboratory (NCL), Pune, India made efforts to justify the machinery for the synthesis gold and silver nanoparticles from various plants such as geranium [28, 76], neem [20], lemon grass [75], tamarind [109] and chickpea [110]. They reported the occurrence of proteins and secondary metabolites in the water soluble fractions of geranium leaves and hypothesized that terpenoid contributes to the reduction of silver ions and oxidized to carbonyl groups. It was also shown that protein was involved in the surface capping of gold nanoparticles synthesized using geranium leaf extract [28]. When pure metallic and bimetallic nanoparticles were synthesized using Azadirachta indica leaf extract, it was observed that reducing sugar in the extract might be responsible for the reduction of metal ions and the construction of consistent metal nanoparticles, and for the stabilization of the nanoparticles, flavanoid and terpenoid could be responsible [20]. The researchers reported that reducing sugars (aldoses) was responsible for the formation of gold nanotriangles by using lemon grass (Cymbopogon flexuosus) [75]. The research group of Gardea-Torresdey et al [111] reported that alfalfa roots are capable of absorbing silver as silver nanoparticles. The TEM/SEM analysis in this study suggested that silver atoms accumulated inside the alfalfa plant tissues undergo nucleation and subsequently form nanoparticles. At the time of Coleus aromaticus leaf mediated production of silver nanoparticles, it was reported that the occurrence of aromatic amine, amide (I) group, phenolic groups, and secondary alcohols may act as reducing agents for the synthesis of nanoparticles [112].
During the synthesis of gold nanoparticles by using fenugreek seed extract, it was observed that fenugreek seed extract performs the dual function of reduction and stabilization of gold nanoparticles [74]. It was elaborated that flavonoids existing in seed extract are potent reducing agents that may be responsible for the reduction of chloroauric acid, whereas the carboxylate group present in proteins can act as a surfactant to attach onto the surface of gold nanoparticles and stabilize them through electrostatic stabilization [74].
After revising all the projected mechanisms so far, we have developed a pictographic diagram showing the conceivable mechanisms behind phytosynthesis of metal nanoparticles (figure 3).
Figure 3. Schematic diagram showing the mechanisms behind the biogenic synthesis of metallic nanoparticles.
Download figure:
Standard image High-resolution image2.3. Factors affecting biosynthesis of nanoparticles
Several factors such as pH, temperature, concentration of plant extract, concentration of metal solution, incubation/reaction time etc, affect the synthesis, size and shape of nanoparticles.
2.3.1. pH
The pH of the medium affects the size of nanoparticles. Available reports indicated that pH is an important factor in the bio-formation of metallic nanoparticles [113]. The study also stated that the size of gold nanoparticles can be controlled by varying the pH of medium [114]. It has been reported that different plant extracts and the extracts coming from different parts of the same plant may have different pH values, nanoparticles synthesized by these different pH extracts have different sizes [93]. It has been stated that larger nanoparticles formed at lower pH (2–4) compared to higher pH [115].
2.3.2. Temperature
One of the most exciting aspects of nanoparticle biosynthesis is the effect of temperature. The temperature of the reaction medium is a serious issue that regulates the nature of nanoparticles formed [115]. When plant extract was used to produce metallic nanoparticles, the percentage of gold nano-triangles relative to spherical particles was significantly reduced at high temperature, whereas low temperature mostly promoted nano-triangle formation [116]. It was also revealed that gold nanoparticles formed with a higher rate at higher temperatures. It was observed that nano-rod and platelet-shaped gold nanoparticles were synthesized at higher temperatures, while spherical-shaped nanoparticles were formed at lower temperatures [117]. Some researchers also reported a rapid synthesis rate of silver nanoparticles at higher temperatures [118]. Therefore, the above studies implied that temperature might be one of the critical issues controlling the size and shape of nanoparticles.
2.3.3. Incubation time
Incubation time is the time duration required for completion of all steps of the reaction. It is revealed that the incubation time also affects the synthesis of nanoparticles. It was deduced that plant mediated synthesis of silver and gold nanoparticles started within 5 min of the reaction but an increase in contact time is responsible for the sharpening of the peaks in both silver and gold nanoparticles [115]. An increase in the sharpness of UV absorption spectra peaks with an increase in contact time was reported while working with chenopodium leaf extract [117]. It was reported that a very slight variation occurred between nanoparticles synthesized within 15 min of the reaction and increased up to 2 h. For the stability of silver and gold nanoparticles, optimum incubation duration is required for complete nucleation and subsequently for the stability of nanoparticles [119].
2.4. Mechanism of antimicrobial action of nanoparticles
The exact mechanism behind antimicrobial activity of metallic nanoparticles is not clearly known and is a contested matter. However, there are many concepts on the action of nanoparticles to cause the antimicrobial effects. Silver nanoparticles have the ability to bind with the bacterial cell wall and consequently penetrate it, thereby causing structural changes in the cell membrane such as the permeability of the cell membrane and the death of the cell. Nanoparticles accumulate on the cell surface, and enter by endocytosis [120]. Free radicals are formed by the silver nanoparticles and it may be considered to be another mechanism for cell death. It was suggested by study of electron spin resonance spectroscopy that there is formation of free radicals by the silver nanoparticles when in contact with the bacteria, and these free radicals have the capability to damage the cell membrane and make it porous which can ultimately lead to cell death (figure 4) [121, 122].
Figure 4. Different possible mechanism of antimicrobial action of nanoparticles.
Download figure:
Standard image High-resolution imageThere is a natural tendency of a soft acid to react with a soft base [123]. The cells are extensively made up of sulfur and phosphorus which are soft bases and silver is a soft acid. DNA has sulfur and phosphorus as its major components; the nanoparticles can act on these soft bases and destroy the DNA which would definitely lead to cell death [124]. The interaction of the silver nanoparticles with the sulfur and phosphorus of the DNA can lead to problems in the DNA replication of the bacteria and thus terminate the microbes.
There is the generation of reactive oxygen species by the contact of silver nanoparticles with bacterial cell which are produced possibly through the inhibition of a respiratory enzyme by silver ions and attack the cell itself [125, 126].
It has also been found that the nanoparticles can moderate the signal transduction in bacteria. There is well-proven information that phosphorylation of protein substrates in bacteria influences bacterial signal transduction. Dephosphorylation is noted only in the tyrosine residues of gram-negative bacteria. The phosphor tyrosine profile of bacterial peptides is altered by the nanoparticles. It was found that the nanoparticles dephosphorylate the peptide substrates on tyrosine residues, which leads to signal transduction inhibition and thus the stoppage of growth. It is, however, necessary to understand that further research is required on the topic to thoroughly establish the claims [127].
2.5. Applications of nanoparticles
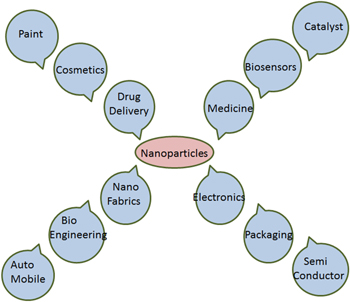
Nanoparticles are of huge interest due to their very small size and large surface-to-volume ratio, and they display absolutely novel uniqueness contrast to the large particles of bulk material [128]. Nanoparticles of noble metals, viz. gold, silver, platinum and palladium, are widely useful in fast-moving consumer goods such as shampoos, soaps, detergents, shoes, cosmetic products, and toothpaste, besides their applications in medical and pharmaceutical products [129].
Silver nanoparticles, due to their unique properties, find use in many day-to-day applications in human life. Silver nanoparticles have been engaged in sensor technology [130], biological leveling, house cleaning chemicals, in fabric cleaners, as antireflection coatings, to improve the transfer of heat from collectors of solar energy to their fuel tanks, to produce high-performance delicate electronics, and many other biomedical applications [131–134]. Though all these are important applications of silver nanoparticles, perhaps their need is most desired in the medical field. The antimicrobial nature of silver nanoparticles is the most exploited nature of silver nanoparticles in the medical field, though the anti-inflammatory nature is also considered immensely useful. Initial studies have suggested that the acceleration of wound healing in the presence of nanoparticles is due to the reduction of local matrix metalloproteinase (MMP) activity and the increase in neutrophil apoptosis within the wound. It has been suggested that the MMP can induce inflammation and hence cause non-healing wounds [135]. A reduction in the levels of pro-inflammatory cytokines was also validated in a mouse model with burn injury when silver nanoparticles were introduced [136]. It was also found that silver nanoparticles can inhibit the activities of interferon gamma and tumour necrosis factor alpha, which are involved in inflammation [137]. Though these studies prove that silver nanoparticles are involved in the anti-inflammatory effects, the exact, precise mechanism of action remains to be determined. Gold nanoparticles have been extensively used in medicine [138, 139], disease diagnostic, drug delivery systems, shows optical properties and electronic properties [140, 141]. Platinum nanoparticles are also extensively used as catalysts [142–145] and in many biomedical applications in combination with other nanoparticles in alloy, core–shell, and bimetallic nanostructure [146]. Similarly, palladium nanoparticles have an extensive application in catalysis and electrocatalysis [91, 147, 148], sensing, and plasmonic wave guiding [149] (figure 5).
Figure 5. Different applications of nanoparticles.
Download figure:
Standard image High-resolution image3. Conclusion
In the field of nano-biotechnology, phytosynthesis of nanoparticles has been performed to create novel materials that are eco- friendly, cost effective, stable nanoparticles with a great significance for wider applications in the areas of electronics, medicine and agriculture. In the current situation nanotechnology inspires progress in all sphere of life, hence the phytosynthetic path of nanoparticles synthesis will emerge as a safe and best alternative to conventional methods. This review has summarized the recent research work in the field of phytosynthesis of noble metal nanoparticles and critically discusses the various mechanisms proposed behind it. Due to the rich biodiversity of plants, their potential for the synthesis of noble metal nanoparticles is yet to be fully explored. Huge numbers of plant species are excellent candidates for nanoparticle synthesis. Elucidation of mechanism behind phytosynthesis of precious metal nanoparticles is necessary in order to develop a rational approach. A detailed understanding of biochemical mechanisms involved in plant-mediated nanoparticle synthesis is a requirement in order to make this approach economically competitive with the conventional methods. Many reports have been published with a proposed mechanism, but most of them are just reasonable hypotheses without any convincing experimental support. Because the hypotheses also vary with various plant species, further in depth evaluation is therefore necessary in order to understand the actual mechanism of a particular plant system. Therefore, this still remains an important issue. Nanoparticles have good antimicrobial activity and in this review we have summarized the possible mechanism behind antimicrobial action of nanoparticles.