Abstract
Presence of acidic, basic and neutral additives on dispersion of multiwalled carbon nanotubes (MWCNTs) in dimethylforamide (DMF) solution has been investigated. The surface charge measurement showed that MWCNTs in the presence of acidic additives in DMF exhibit a higher surface charge (−85 C g−1) than that with the basic additives (−22 C g−1). The stability of the MWCNTs dispersion was visually monitored and it was found that in the presence of acidic and no additives it would be stable and dispersed for more than five days, whereas MWCNTs suspension immediately settle down in the presence of basic and neutral additives. The degree of defects on MWCNTs was determined by analysis of detailed Raman spectra of as-received MWNCTs and MWNCTs dispersed in DMF with different additives. By exploring the correlation between the ID/IG (Raman analysis) ratio and the degree of defects, it was found that the carbon–carbon double bond (C=C) of MWCNTs was slightly damaged by adding additives to the solvent.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Pristine carbon nanotubes (CNTs) and more specifically multiwalled carbon nanotubes (MWCNTs) attract scientific interest because of their unique electrical, mechanical and optical properties. They show a wide range of potential applications in biosensors [1], composite [2], field emission devices [3], electronic components [4] and probe tips [5]. One of the main challenges in the research field of CNTs is the dispersion and stabilization in different solvent media and also in polymer matrices. It is conducting due to delocalization of π-electrons, whereas there is a possibility of adsorption of various chemical groups via π−π stacking interaction [6]. Unfortunately, a high aspect ratio makes nanotubes aggregate easily and it is difficult to suspend them as a result of substantial van der Waals attractions between tubes. According to Girifalco et al [7]. CNTs are bundled with strong van der Waals interaction energy of 500 eV μm−1 of tube–tube contact. Such high interaction energy makes CNTs dispersion a challenging task.
There are two methods for dispersing CNTs: (i) chemical method and (ii) mechanical method. In the chemical method the surfaces of CNTs are functionalised with an easily ionisable group such as –COOH, –OH which enhances the hydrophilicity of their surfaces as well as electrostatic repulsion between them. This is achieved by using strong acid at high temperature, but that introduces structural defects in CNTs. In the mechanical method, it is essential to use high shear mixing, ultrasonication and to choose a suitable solvent. According to Cheng [8] the dispersion and debundling of CNTs in organic solvents is critically dependent on the sonication process, which is closely dependent on many physical parameters of the solvent, including vapour pressure, viscosity, surface tension, density and molecular weight. It is furthermore well established that high intensity mechanical mixing causes damage to the nanotubes and choice of solvent should be guided by minimizing mechanical mixing requirements.
Dimethylformamid (DMF) is a well-recognized dispersant [9–12] for CNTs and for processing of nanocomposites. Chemical structure of DMF is shown in figure 1. Fawad et al [13] reported that DMF is a good dispersant for preparing homogeneous and agglomerate-free slurries in order to fabricate ceramic-CNTs composite. Lau et al [14] studied the solvent effect on the dispersion behaviour of CNTs and concluded that DMF more effectively disperses CNT in polymer matrix than its counterparts ethanol and acetone. Kang et al [15] reported an alternative, noncovalent method of modifying SWCNTs by encasing the nanotubes within cross-linked, amphiphilic copolymer micelles by using different H2O: DMF ratio and concluded that the total yield of isolated SWCNTs product clearly depends on H2O: DMF ratio.
Figure 1. Molecular structure of dimethyl formamide (DMF).
Download figure:
Standard image High-resolution imageDMF is a widely used solvent in various fields such as the pharmaceutical industry, polymer chemistry, synthetic organic chemistry and electrochemistry. Considering its application in the pharmaceutical industry, it is very important to study the effect of acid, bases and neutral additives in the dispersion as well as chemical properties of MWCNTs. The acidic and basic additives in any medium highly influence charging of molecules and hence their dispersion properties. Thus, the stability of CNTs dispersions is also influenced by pH and the salt concentration of the environment. When they are used for electrical purposes, these aspects must be studied in detail. To date no one has reported the effects of acidic, basic and neutral additives on the dispersion behaviour of CNTs molecules in organic medium.
In this work we report on the dispersion behaviour of pristine MWCNTs in DMF solution in the presence of acidic, basic and neutral additive. Visual observation, UV–Vis spectroscopy and scanning electron microscopy (SEM) were used to study the dispersion of the CNTs in the solution. The stabilization mechanisms of the CNTs dispersion are discussed by zeta potential and surface charge measurement. The defects on the surfaces of the CNT samples are characterized by Raman spectroscopy.
2. Materials and methods
2.1. Materials
All the chemicals used were of analytical grade. The MWCNTs were produced by Sigma-Aldrich Chemie, GmbH, Steinheim, Germany, (outer diameter 110–170 nm, length 5–9 μm) and were used as-received. The DMF was purchased from Merck. All solutions were prepared in deionized water with resistivity 18.2 MΩ cm. HCl was used as the acidic, NaOH as basic and deionized water as neutral additives throughout the experiment.
2.2. Methods
MWCNTs-DMF suspension of 0.01 wt% was prepared by ultrasonication of output power of 50 W for 30 min and was marked as master suspension. To perform the DMF dispersion of MWCNTs suspension with acid additive, 1 ml of the 0.1 M aqueous solution of HCl was added to 9 ml of master suspension. Similarly, DMF dispersion of MWCNTs with basic additive was performed with the addition of 1 ml of 0.1 M sodium hydroxide aqueous solution in 9 ml of master suspension. Finally, 1 ml of deionised water added in 9 ml master suspension represented DMF stabilized MWCNTs in the presence of neutral additive. The master suspension itself represented as DMF stabilised MWCNTs without additive. To prepare the sample for micro-structural characterization, respective MWCNTs suspension was sonicated for 30 min followed by centrifuge at 2000 rpm for 10 min. The supernatant solution was collected and used for SEM and TEM slide preparation.
2.3. Techniques
2.3.1. Electron microscopy measurements
The dispersions of MWCNTs were characterized using SEM, (Hitachi-3400N, Tokyo, Japan), transmission electron microscopy (TEM) (FEB Tecnai G2 20, Netherlands). A drop of 15 μl of the respective MWCNT suspension prepared by the method described in section 2.2 was dropped onto glass slide for SEM and on carbon coated TEM grids (300 mesh, 3 mm, purchased from TAAB Laboratories, UK) for TEM and allowed to dry. The slides/grids were then examined under SEM and TEM.
2.3.2. Surface charge measurements
The specific surface charges of aqueous and organic disperse MWCNTs were measured with a particle charge detector (PCD; model PCD-04-pH, Germany). It makes use of measurement of streaming potential which develops due to partial shearing of counter ions of the electrical double layer from the charged particles during the relative flow of liquid. The working principle of PCD and further experimental details are described elsewhere [16, 17].
2.3.3. Stability measurements
A dispersion characteristic of the MWCNTs-DMF suspension was carried out using standard vials of 10 ml. The MWCNTs-DMF suspensions with and without additives were stirred thoroughly and ultrasonicated (50 W, 43 kHz) for about 30 min for proper mixing and allowed to stand undisturbed. Suspension stability was noted visually and recorded optically with time. Further, the dispersions of MWCNTs in DMF were characterized using UV–Vis spectrophotometer (UV-2450, Shimadzu) operating between the ranges of 200–800 nm. In the first set of experiments, baseline correction was carried out using pure DMF solvent then absorption characteristic was measured at different intervals of time.
2.3.4. Raman spectroscopy
Raman spectroscopy was used to investigate the interaction between MWCNTs and organic solvent (Invia Reflex/514, Incoterm, UK) with incident argon laser excitation wavelength of 514 nm. Raman spectrum is taken after drop casting the solution on a glass slide and evaporating the organic solvent in the range of 500–3500 cm−1.
3. Results and discussion
3.1. Morphology of the MWCNTs dispersion
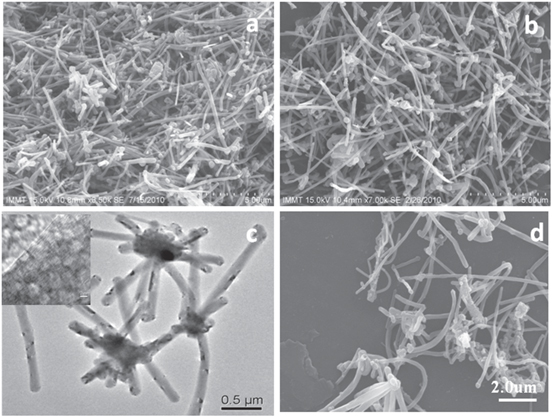
The morphology of the as-received MWCNTs powder using SEM micrograph shows heavily cross-linked, rope-like entangled bundles (figure 2(a)). Powder dispersed in water did not reduce any entanglement of MWCNTs (figure 2(b)). A TEM image of nascent MWCNTs, as shown in figure 2(c) also confirms a considerable degree of entanglement. Figure 2(d) shows the SEM micrograph of MWCNTs dispersed in ethanol with some degree of agglomeration.
Figure 2. SEM images of MWCNTs (a) as-received, (b) dispersed in water (c) TEM micrograph of MWCNTs, with MWCNTs at higher resolution (inset) and (d) SEM of MWCNTs dispersed in ethanol.
Download figure:
Standard image High-resolution imageFigure 3 shows the optical image of the MWCNTs-DMF suspension in the presence of three different additives (acidic, neutral and basic) and without additive (master suspension). The MWCNTs-DMF suspension in the presence of basic additive was observed to settle at the bottom of vials immediately (2nd vials of figures 3(b)–(d). In the case of MWCNTs-DMF suspension with neutral additive, the aggregation was retarded and suspension settled completely after five days (1st vials figure 3(d)). As visually demonstrated in figure 3 (3rd vials b, c and d), DMF helped improve the CNT dispersion for more than five days. In the presence of acidic additive, dispersion remained stable after five days (4th vials of figures 3(b)–(d)).
Figure 3. Optical images show dispersion of MWCNTs in DMF with neutral additive (1st bottle) and with basic additive, without additive and in acidic additive (2nd, 3rd and 4th vials) after (a) 0 h, (b) 1 h, (c) 1 day and (d) 5 days.
Download figure:
Standard image High-resolution imageThe SEM images (figure 4(a)) of MWCNT powder dispersed in DMF clearly show that the CNTs molecules are well separated from each other. There is minimization of bundle formation in the presence of acidic and neutral additives (figures 4(b) and (c)). However, in the presence of basic additive, bundles formation started (figure 4(d)). Poor CNTs dispersions in the presence of basic additive in DMF may be associated with high ionic strengths originating from the addition of NaOH used as basic additive. In these cases, a sizeable amount of NaOH was added and the high content of sodium cations led to high ionic strength; this causes the aggregation of MWCNTs by ionic bonding, leading to precipitation [18].
Figure 4. SEM micrograph of MWCNTs dispersed in DMF (a) without additives, (b) acidic additives, (c) neutral additives and (d) basic additives.
Download figure:
Standard image High-resolution image3.2. Colloidal stability of the MWCNTs dispersion
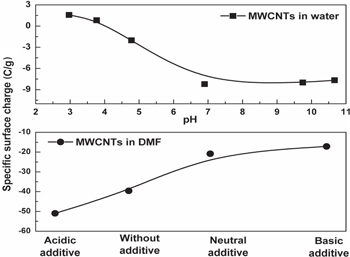
When MWCNTs are dispersed in solvent such as water, ethanol or DMF, the particles are highly influenced by a variety of forces including forces of gravity, drag force, van der Waal forces etc, from the medium; as a result the surface charges varies. So, the determination of the surface charge of MWCNTs is of immense importance to knowing the dispersion characteristics in a particular solvent. The stability of dispersion is controlled by the interactions between particles. One can suppress particle agglomeration and increase the stability of MWCNTs dispersion by enhancing the inter-particle repulsive forces through increase in surface charges on the particles. Figure 5 shows the results of surface charge measurement of pristine MWCNTs dispersed in aqueous medium and in DMF (organic medium) in the presence of different additives. The aqueous dispersion of native MWCNTs shows slightly positive surface charges in acidic pH and slightly negative charges in alkaline pH and the isoelectric point (pHiep) is located at pH 3.9. As the magnitude of surface charge in normal pH is very low, MWCNTs get agglomerated rather than dispersed as shown in figure 2(b).
Figure 5. Specific surface charge measurement of MWCNTs in water and organic suspension.
Download figure:
Standard image High-resolution imageWhen MWCNTs are dispersed in DMF, it acquires high negative charge (figure 5). The overall surface charge is decreasing with changing acidic to basic condition, and practically no isoelectric point can be observed. DMF is a highly polar protophilic stable solvent and neutral hydrolysis of DMF is practically impossible at room temperature due to higher activation free energy barrier (55 Kcal mole−1) [19, 20], whereas acid and base catalysed hydrolysis need only 20 and 14 kcal mol−1, respectively, to overcome this activation energy barrier. Hence, in the presence of acid and alkali, DMF gets partially hydrolysed to formic acid and dimethylamine. Also, it is reported that the neutral hydrolysis of formamide does not take place at all due to higher barrier energy, so here DMF undergoes dilution in the presence of water [20]. This is clearly explained in the schematic diagram (figure 6).
Figure 6. Schematic representations of the mechanism by which additives help to disperse MWCNTs in DMF.
Download figure:
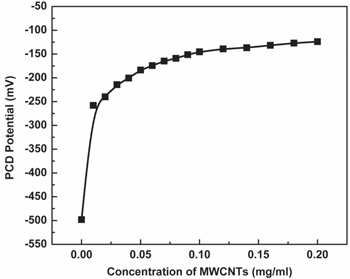
Standard image High-resolution imageMWCNTs are hydrophobic in nature and wetting of MWCNTs with DMF happens through hydrophobic interaction of alkyl chain with the side walls as represented in the schematic diagram (figure 6). In acidic environment, as mentioned earlier, DMF will undergo partial hydrolysis to formic acid and dimethylamine, but further formic acid will ionize to produce carboxylic moieties (pKa value of HCOOH is 3.7). Undissociated DMF will interact through hydrophobic interaction with MWCNTs sidewall as before, whereas carboxylic moieties will attach themselves at the defect sites. As a result, in the presence of acidic additive, MWCNTs develops very high negative charges (−85 C g−1). In only DMF, undissociated DMFs interact with the MWCNTs sidewall through hydrophobic interaction and hence develop fairly high negative charges (−39.6 C g−1). Addition of water will dilute the concentration of DMF and, as before, undissociated DMF will interact with MWCNTs. Hence, the suspension remains fairly stable but in the long run, it settles since water does not wet MWCNTs. Also, surface charges reduce to less negative value (−20.8 C g−1). In the presence of alkaline additive, DMF will dissociate to formic acid and dimethyl amine, however, extent of formic acid ionization will be less. Interaction of some undissociated DMF will result in some negative charges (−17.4 C g−1) but suspension is very unstable and is found to be settling within 1 h. The reason for highly unstable suspension even though there is fairly good amount of negative surface charges in MWCNTSs may be due to high ionic concentration of solvent. It has been shown [21] that high ionic concentration tends to suppress the extent of double layer overlaps of neighbouring MWCNTs that is essential for stabilization. Figure 7 shows the variation of streaming potential as a function of MWCNTs concentration. As the concentration of MWCNTs increases, streaming potential increases and at a particular concentration (0.1 mg ml−1) it reaches maximum. Further addition of MWCNTs does not change the streaming potential. At an optimum concentration of 0.1 mg ml−1 of MWCNT, available DMF covers the MWCNTs surfaces completely, forming a DMF mono layer. At higher concentration, MWCNTs remain uncovered and hence streaming potential remains unchanged.
Figure 7. Determination of stability maxima of MWCNTs in DMF.
Download figure:
Standard image High-resolution image3.3. Characterization of the MWCNTs dispersion
MWCNTs are inactive in the wavelength region between 200–800 nm since the photoluminescence properties of nanotubes are quenched due to carrier tunneling [22] in this region. So it is possible to characterize the dispersion of MWCNTs in organic solvent using UV–Vis spectroscopy and where absorbance values were recorded at 260 nm as reported in previous studies [12, 23, 24].
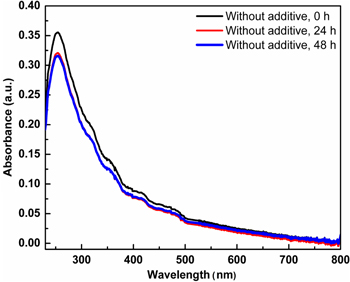
As illustrated in figure 8, the UV–Vis spectrum of the MWCNTs in the DMF solution ultrasonicated (0.1 mg ml−1, 30 min sonication at 50 W, 43 kHz) obtained after different storage times shows the slight decrease of absorption intensity after 24 h that indicates the precipitation of carbonaceous, nano graphite impurities. But after 48 h there is no change in the intensity of absorption band confirming good dispersion of MWCNTs in DMF solvent. Further, we characterized MWCNTs dispersed in DMF in the presence of different additives (acidic, aqueous and alkaline) at different storage times, by UV–Vis absorption spectroscopy. Figure 9 shows the absorption intensities at 260 nm of DMF dispersed MWCNTs at different storage times having acidic, neutral and alkaline additives.
Figure 8. UV–Vis absorption spectrum of MWCNTs suspension stabilized by DMF (master suspension) at different storage times.
Download figure:
Standard image High-resolution imageFigure 9. The absorption intensities at 260 nm of MWCNTs suspension stabilized by DMF at different storage times.
Download figure:
Standard image High-resolution imageIn acidic additive, the intensity of absorption peak changes very slightly at different storage times confirming the stability of suspension in acidic medium, whereas in the presence of alkaline additive, the intensity of absorption peak decreases drastically after 24 h confirming the destabilisation of MWCNTs suspension, with neutral additive; suspension is slowly destabilised.
To elucidate any damage caused by the dispersion procedure of MWCNTs in a particular solvent, we studied the Raman spectra of pristine MNCNTs sample and dispersed MWCNTs sample under the same conditions (figure 10). The MWCNTs display three characteristic bands at ∼1353 cm−1, ∼1575 cm−1 and 2696 cm−1. The first 'D' band at ∼1353 cm−1 is derived from disordered graphite structures, while the second band at 1575 cm−1 is assigned to the 'G' band, caused by the vibration of C–C bonds in the graphitic structure [25, 26], and the third band at 2696 cm−1 is from a photon–second phonon interaction, apart from a week band at around 2447 cm−1, which is due to high order pyrolytic nature of carbon materials. After dispersion, these characteristic peaks are still present, proving that the dispersion procedure with different solvents does not damage the structure of MWCNTs.
Figure 10. Raman spectra of as received MWCNTs and MWCNTs dispersed in with different additives.
Download figure:
Standard image High-resolution imageIt has been reported that dispersion by adding additive to the solvent not only exfoliates the MWCNTs bundles but can also induce defects and even scission of the tubes [27]. The damage of the MWCNTs is normally checked by Raman spectroscopy, where the ratio of the intensities of defect or 'D' band compared to graphitic or 'G' band gives a measure of damage to the tubes [28, 29].
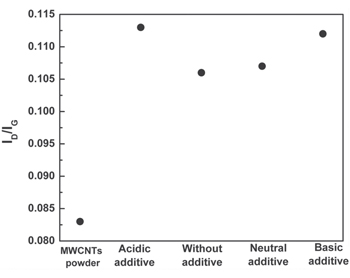
Figure 11 shows the variation of ID/IG ratio as a function of different additives. The ratio increases significantly in the case of dispersed MWCNTs as compared to pristine MWCNTs. It shows that the dispersion procedure introduces defects on the walls of MWCNTs [30]. The ID/IG ratio in acidic and alkaline additive is higher than that without additive and with neutral additive. This means that more damage is created if any acid or base is added to the solvent, and more surface charges are generated on MWCNTs.
Figure 11. ID/IG ratio as a function of different additives.
Download figure:
Standard image High-resolution image4. Conclusion
The dispersion and debundling of MWCNTs in DMF organic solvents is somehow dependent on types of additive present in the solvent. Without adding any additive to DMF and in the presence of acidic additive, we are able to get dispersed MWCNTs suspension for more than five days, whereas in alkaline additive it will immediately start to settle down in the bottom of the vials. It is furthermore clear that even in the presence of acidic additive the MWCNTs showed excellent dispersion, but this results in damage to the nanotubes and hence, choice of additive should be guided by minimizing damage on nanotube walls. Changing surface charges by adding any additive to solvent clearly causes progressive physical or chemical modification or defects of the MWCNTs. The investigation of the stabilization mechanism in the presence of different additives can help provide a guideline for choosing a suitable additive to prepare homogeneous MWCNTs dispersion for various applications. Therefore, it is concluded that DMF is a good dispersant for preparing homogeneous and agglomerate-free slurries by any type of colloidal processing.
Acknowledgements
The authors would like to acknowledge CSIR-IMMT, Bhubaneswar and GERMI Research, Innovation and Incubation Centre, Gandhinagar for support.