Abstract
We have studied the photochromic reaction of the diarylethene derivative on Au nanoparticles using the incoherent excitation as a function of the wavelength of the irradiation light with the aim to clarify the effect of metal nanoparticles on the reaction yield. The photochemical reaction was suppressed by the Au nanoparticles under the irradiation of light whose wave length was shorter than 700 nm, while photochemical reaction was enhanced by the irradiation of light whose wavelength was longer than 750 nm via two-photon absorption process. The suppression of the photochemical reaction could be explained by the quenching of the excited state via radiative and non-radiative decay through energy or charge transfer to the metal substrate (e.g. electron–hole pair formation, surface plasmon excitation, formation of induced-dipole induced-dipole coupling), and the absorption of light by the Au nanoparticle.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Photochemical reaction on metal nanoparticles has attracted increased attention due to its high throughput and low energy requirement. Localized plasmon on the metal nanoparticles induces a reaction with lower energy in the rage of visible light. Up to now, various interesting plasmon-induced reactions have been reported. Adleman et al [1] reported the light induced evolution of CO2, CO and H2 gas from the liquid mixture of water and ethanol over Au nanoparticles in a microfluid channel. Here, the plasmon heating of the nanoparticles provides the heat of the reaction. Mukherjee et al reported the room temperature dissociation of H2 on Au nanoparticles using visible light. Surface plasmon excited in the Au nanoparticles decays into hot electrons, which can transfer into an H2 molecule, triggering dissociation [2]. Tsuboi et al reported that a photochromic reaction can be driven by irradiation of near infrared laser light. The yield of the photochromic ring-opening reaction was proportional to the square of the intensity of the light source, which explained the progression of the two-photon reaction [3–6]. The origin of the two-photon reaction is an enhancing effect that acts on the electromagnetic field of the Au nanoparticles. Two-photon polymerization of a photoresist polymer was also reported on Au nanoblocks [7–10]. Without metal nanoparticles, the photochemical reaction mentioned above requires light with much higher energy.
The existence of the Au nanoparticles can induce the photochemical reaction with low energy light. On the other hand, the photochemical reaction can be suppressed by the existence of metal close to target molecules in some cases. The quantum yield for the ring closing reaction of diarylethenes decreases from 40% to 7% by connecting the molecule to the Au nanoparticles in solution [11]. In a single molecular junction study where a photochromic molecule is chemically bounded to two Au electrodes (particles), switching of the photochromic molecule from the open form to closed form does not proceed on Au, while the switching of the photochromic molecule from the closed form to open form proceeds [12]. The decrease of the quantum yield of photodissociation of molecules was reported on metal surface including CH3I chemisorbed on Ag(111), and Pt(111), for CH3Cl on Ni(111) [13–17]. The suppression of photochemical reaction has been explained by the quenching of the photo excited state, mixing of the excited state and Au Fermi level, and by other reasons.
In the present work, we have studied the photochemical reaction of diarylethene (DE) derivative on Au nanoparticles as a function of the wavelength of the irradiation light, in order to reveal the positive and negative effect of existence of metal nanoparticles mentioned above. This system is an ideal one to study the effect of metal nanoparticle, because the progress of two-photon reaction is well known and experimental procedure including sample preparation is well-established [3, 4, 18]. Murakami et al [18] showed DE's ring-opening reaction is induced via two-photon absorption process using femtosecond laser method. Tsuboi et al have studied DE derivatives on Au nanoparticle using a near-infrared continuous wave laser light. The progression of the two-photon reaction was confirmed by the analysis of the reaction yield as a function of irradiation intensity [3]. In this study we have revealed that the photochemical reaction was suppressed by the Au nanoparticles with the irradiation of light whose wavelength was shorter than 700 nm, while photochemical reaction was enhanced by the irradiation of light whose wavelength was longer than 750 nm via two-photon absorption process.
2. Experimental
The Au nanoparticles were deposited on a quartz substrate by a silane coupling agent [3]. Quartz substrates were cleaned by immersion for one day in piranha solution (H2SO4 (Wako):H2O2 (Kanto Chemical) =7:3), rinsed with pure water twice and then dried by blowing argon gas. The substrate was soaked in 10% (v/v) ethanol (Kanto Chemical) solution of (3-aminopropyl)trimethoxysilane (Tokyo Kasei) for 3 h and washed twice with pure water and ethanol, and dried by blowing argon gas. In this process, the silane coupling agent covalently binds to the quartz surfaces by means of hydrolysis and anime-terminated silane monolayer forms on the surface. Finally, the Au colloidal solution (Aldrich, diameter = 20 nm) was dropped on the substrates and the solvent was evaporated in air to fix the Au nanoparticles. After evaporation, the substrates were washed with pure water (resistance >18 MΩ, mili-Q) and ethanol, and dried by blowing argon gas. The colour of Au nanoparticles on the quartz substrates (Au nanoparticle substrate) was light blue. Since the amino groups have affinity to gold, the Au nanoparticles were immobilized on the quartz substrates. Au nanoparticle substrate was characterized using the ultraviolet-visible (UV–vis) extinction spectrometer (JASCO Co. V-650) and observed using the scanning electron microscope (SEM).
The acetonitrile (Wako) solution of 1,2-bis(2,4-dimethyl-5-phenyl-3-thienyl)-3,3,4,4,5,5-hexafluoro-1-cyclopentene (DE, Tokyo Kasei) and poly(methyl methacrylate) (PMMA, Aldrich) were spin-coated (2 s for 400 rpm and then 30 s for 1200 rpm) on the Au nanoparticle substrates to form a thin film. The concentrations of DE and PMMA were 1.4 × 10−2 M and 1.0 wt%, respectively. Afterwards, the substrates were irradiated with incoherent light using a Xe lamp (Hamamatsu Photonics Co.) at room temperature. Band-pass or high-pass filters were used to select the desired wavelength of light. The source intensity was 5–25 mW cm−2. The photochromic reaction of DE was monitored by UV–vis extinction spectra.
3. Results and discussion
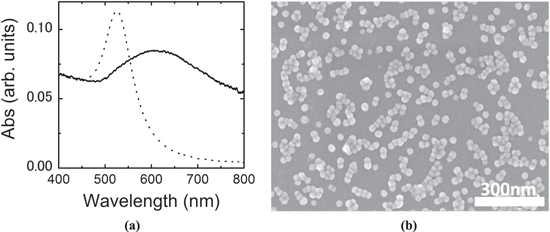
Figure 1(a) shows the UV–vis extinction spectra of Au nanoparticle substrate, together with that of the mother colloidal solution. The mother colloidal solution exhibited an absorption band around 550 nm due to the localized surface plasmon of the Au nanoparticles. The Au nanoparticle substrate exhibited a broad peak around 600 nm and a broad shoulder absorption band extending to the near infrared region (600–900 nm). The red shift of the absorption peak and appearance of the shoulder indicate the presence of electronic interactions among the Au nanoparticles. Figure 1(b) shows the SEM image of the Au nanoparticle sample. Au nanoparticles were inhomogeneously aggregated, which produced nano gaps among adjacent nanoparticles around which electromagnetic fields of incident light can be much enhanced.
Figure 1. (a) UV–vis extinction spectra of Au colloid solution (dotted) and Au nanoparticle sample (solid), (b) SEM image of the Au nanoparticle sample.
Download figure:
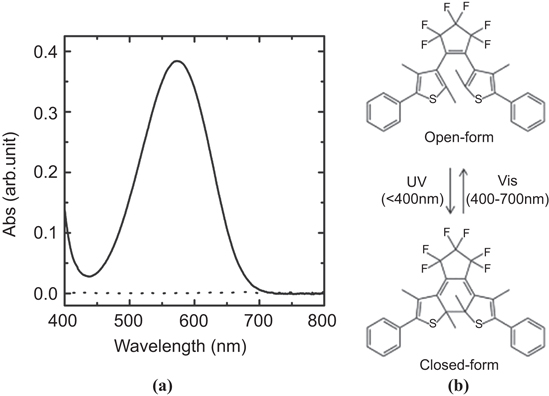
Standard image High-resolution imageFigure 2 shows the UV–vis extinction spectra of DE in open and closed forms in the solution. The 600 nm peak was observed for the closed form, while there was no feature for the open form. DE undergoes reversible ring opening and closing reactions induced by visible and UV light irradiation, respectively [19, 20].
Figure 2. UV–vis extinction spectra of DE in open (dotted) and closed (solid) forms (a), together with the molecular structure of DE (b).
Download figure:
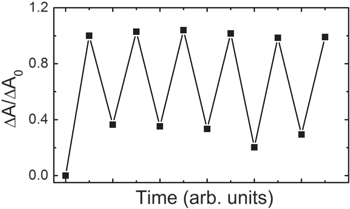
Standard image High-resolution imageFigure 3(a) shows the UV–vis extinction spectra for the DE films on Au nanoparticle substrate. It is noted that the absorption peak of the DE in closed form is overlapping the absorption peak due to the localized surface plasmon of the Au nanoparticles in the UV–vis extinction spectra. The absorption spectra (figure 3(b)) of the DE film were obtained by subtraction of the spectrum of the open form of DE on Au nanoparticles from the observed spectrum of the sample at each irradiation time. Before irradiation, a band was observed around 580 nm, and these can be assigned to the closed form of DE. Decrease in the absorbance corresponds to the occurrence of the ring-opening reaction. The absorption band due to the closed form of DE decreased with irradiation time. The band recovered to its starting value by irradiation with UV light (UV lamp, irradiation for several minutes). Figure 4 shows the trace of absorption changes for the DE derivative on Au nano particle switched by repetitive UV–vis light irradiation. The absorption was normalized by that of the initial value. Absorption at 600 nm dropped upon the irradiation of light whose wavelength was longer than 510 nm and recovered upon UV irradiation. This cycle proceeded repetitively, and it was obvious that the absorption drop and recovery were due to the ring-opening and ring-closing reactions, respectively.
Figure 3. (a) UV–vis extinction spectra of DE film on Au nanoparticle substrate after irradiation of light (∼500 nm) for 0 (light gray), 5 (gray), 10 (dark gray), and 20 min (black). The inset shows magnified spectra near the peak position. (b) The spectra of the DE film. The spectra were obtained by subtraction of the spectrum of the substrate itself from the observed spectrum of the sample at each irradiation time.
Download figure:
Standard image High-resolution imageFigure 4. Trace of absorption change at 600 nm of the DE derivative on Au nano particle induced by repetitive irradiation with visible (>510 nm) and UV lights. The absorption was normalized by the initial absorbance.
Download figure:
Standard image High-resolution imageWe then investigated the ring-opening reaction of DE film on Au nanoparticle substrate as a function of wavelength of the source. Figure 5(b) shows the peak intensity in the UV–vis extinction spectra of DE film on Au nanoparticle substrate, where the wavelength of the source was longer than 750 nm. The decrease in the peak intensity indicates the progression of the ring-opening reaction of DE. In condition (solution), the ring-opening reaction of DE does not proceed with the light whose wavelength is longer than 750 nm. The progression of the ring-opening reaction can be explained by the two-photon reaction, which agrees with the previous study [3]. On the Au nanoparticle substrate, nanometer scale gaps are formed between Au nanoparticles. Localized surface plasmon formed in the Au nanoparticle interacts with that in the Au nanoparticle in the vicinity, which leads to the localization of light wave into gap regions and providing a strong enhancement of near field light. The intensity of the near field light can be as high as ∼104 times greater than the incident light field [9]. Due to the high intensity of light, absorption of a second photon of light is possible. Therefore, two-photon photochemical reaction can proceed on the Au nanoparticle substrate due to the electromagnetic field enhancement effects of metallic nanoparticles.
Figure 5. Peak intensity in the UV–vis extinction spectra of DE film on Au nanoparticle substrate (black) and on quartz substrate (white) as a function of irradiation time. The wavelength of the source was (a) 500 nm and (b) more than 750 nm.
Download figure:
Standard image High-resolution imageIn contrast, the ring-opening reaction of DE film was suppressed by the presence of the Au nanoparticle, when the wavelength of the source was shorter than 700 nm. Figure 5(a) shows the peak intensity in the UV–vis extinction spectra of DE film on Au nanoparticle substrate and on quartz plate as a function of irradiation time. The reaction rate was slower on the Au nanoparticle substrate than on the quartz plate. Here, the wavelength of the source was shorter than that required to convert from the closed to open form of DE. Similar suppression of the photochemical reaction has been observed on the metal surface, as discussed in the introduction. The suppression of the ring-opening reaction of DE film can have several grounds. Excited molecule can be quenched near a metal surface via radiative and non-radiative decay through energy or charge transfer to the metal substrate (e.g. electron–hole par formation, surface plasmon excitation, formation of induced-dipole induced-dipole coupling) [13, 21–24]. The mixing of the Au states and the first excited molecular electronic state can also result in quenching of the excited molecular state [12]. Another possibility is the absorption of light by the Au nanoparticle. In the present experimental system, some DE molecules may get behind Au nanoparticles under light irradiation. Some part of the incident light is adsorbed before reaching the DE molecule. The ring-opening reaction rate is suppressed, caused by the decrease in the light intensity.
4. Conclusion
In conclusion, the photochromic reaction of the DE film on Au nanoparticles was investigated as a function of wavelength of the light. Depending on the wavelength used for the photochromic reaction, the presence of the nanoparticle made positive and negative impact on the reaction yield. The photochemical reaction was suppressed by the Au nanoparticles with the irradiation of light whose wavelength was shorter than 700 nm, while photochemical reaction was enhanced by the irradiation of light whose wavelength was longer than 750 nm. The enhancement of the photochemical reaction was explained by the two-photon reaction. Strong electric field is formed in the nanometer scale gaps formed between Au nanoparticles. The two-photon reaction proceeded due to these electromagnetic field enhancement effects. On the other hand, the suppression of the photochemical reaction could be explained by the quenching of the excited state via radiative and non-radiative decay through energy or charge transfer to the metal substrate, and/or the absorption of light by the Au nanoparticle.
Acknowledgments
This work was supported by a Grant-in-Aids for Scientific Research in Innovative Areas (No. 24245027, 25104710, 25620055, 25104710, 26102013), a Grant-in-Aid for Scientific Research (A) (No. 24245027) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and Asahi glass foundation. The authors would like to express their sincere gratitude to professor Y Tsuboi for useful discussion. The authors thank the Center of Advanced Material Analysis in Tokyo Institute of Technology.
Footnotes
- *
Invited talk at the 7th International Workshop on Advanced Materials Science and Nanotechnology IWAMSN2014, 2-6 November, 2014, Ha Long, Vietnam.