Abstract
In this study UiO-66 and UiO-66-NH2 were synthesized by solvothermal method. The effect of preparation conditions on the quality of UiO-66-NH2 was studied. The obtained material has been characterized by x-ray diffraction (XRD), infrared spectroscopy (IR), thermogravimatric analysis (TGA), scanning electron microscopy (SEM) and nitrogen physisorption measurements (BET). The CO2 and CH4 physisorption measurements were carried out using a high pressure volumetric analyzer (Micromeritics HPVA—100). The results showed that the UiO-66-NH2 of ball shape crystalline had been obtained and characterized by high surface area (BET) up to 876 m2 g−1, specific volume 0.379 cm3 g−1, pore radius 9.5 Å and thermal stability up to 673 K, respectively. The experiments indicated that in comparison with UiO-66 the addition of NH2 is able to increase the CO2 and CH4 storage capacity at 1 bar and 303 K twice from 28.43 cm3 g−1 up to 52 cm3 g−1 and from 6.68 cm3 g−1 to 11.1 cm3 g−1, respectively.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The emission of CO2 leads to adverse impacts on the environment such as global warming, rising of sea-level as well ocean acidity. The CO2 and CH4 are currently the major source of emissions leading to greenhouse effect [1]. Therefore, there are many researches focused on removal of these gases from the environment. Among methods used for this purpose, adsorption and separation by porous materials is shown to have high yield and to be economically competitive. A good adsorbent should have large specific surface, high porosity, flexible frame structure and periodic arrangement for the reversible adsorption and desorption process [2]. However, some traditional adsorbents such as zeolites, activated carbons, calcium oxides, hydrotalcites amino etc, are very difficult to regenerate at low temperature, then adsorption capacity of adsorbents will decrease from time to time, resulting in high cost of treatment [3, 4]. Recently, metal organic framework (MOFs) have become high potential materials for storage and separation of gases thanks to their high specific surface area, large pore volume, molecular cell and homogeneous structure, adjustable chemical functionality [5–9]. The adsorption of CO2 in MOF-2 was first studied by Yaghi et al [10]. Then, the CO2 storage capacity in nine MOFs at room temperature and 42 bar was also tested [11]. In other research, the result showed that adsorption capacity of CO2 on MOF-177 at 35 bar was reached 33.5 mmol g−1, nine times higher than the amount of CO2 in a container without adsorbent [11]. The adsorption capacity of CH4 on MOFs was also studied and it reached 155 cm3 g−1 on IRMOF-6 at 35 bar [12]. Mueller et al have synthesized MOF-210 with high specific surface area which reached 10 400 m2 g−1 with CH4 storage capacity of 666.82 cm3 g−1 CH4, that was higher than other porous materials [13]. Although the adsorption ability of CO2 and CH4 on MOFs is confirmed, the important feature of MOFs is their thermal and water stability. Previous investigations showed that although adsorption capacity of UiO-66 is not high compared to other MOFs, the advantage of UiO-66 consists in its structural stability in water and high thermal stability [14].
Adding functional groups in structures is one of the methods to increase the adsorption capacity of MOFs. Therefore, UiO-66-NH2 was synthesized and its physical and chemical properties are also studied. The effect of preparation parameters such as solvents volume, reaction times, exchange solvents and activated times on the propertiy of MOF were also investigated. The adsorption capacity of CO2 and CH4 on UiO-66-NH2 are obtained and compared to UiO-66.
2. Experimental
UiO-66 and UiO-66-NH2 were prepared following the procedure described by Luu Cam Loc et al [15] and Kandiah et al [16]. In order to determine the optimal condition of UiO-66-NH2 preparation, in this study the volume of solvent was varied from 10 ml to 30 ml, reaction time was varied from 12 h to 48 h and activation time was varied in the range of 3 h to 9 h at a temperature of 473 K.
The morphologies of the synthesized product were obtained using a scanning electron microscope (SEM, HITACHI S−4800). Powder x-ray diffraction (PXRD) data were recorded on a Bruker D8 Advance diffractometer. Thermogravimetric analysis (TGA, Q500 V20.10) was also performed on samples to study the thermostability of the materials. Brunauer–Emmett–Teller (BET) surface areas and total pore volumes of the samples were determined from N2 adsorption isotherms at 77 K on Quantachrome NovaWin machine. Fourier transform infrared (FT-IR) spectra (4000 − 400 cm−1) were obtained from KBr pellets using a Bruker Tensor 22 instrument.
The gas (CO2, CH4 and mixture 50%CO2/50%CH4) adsorption capacity on UiO-66 and UiO-66-NH2 was investigated using a high pressure volumetric analyzer (Micromeritics HPVA-100) at temperature 303 K and pressure in range from 1 to 30 bar.
3. Results and discussion
3.1. UiO-66-NH2 synthesis
3.1.1. Effect of reaction solvent volume
It can be observed from XRD patterns (figure 1(a)) that for the UiO-66-NH2 synthesized in different solvent volumes, the highest intensity of characteristic peaks is obtained in the case of 16 ml solvent. The reason may be that the volume of solvent is too little (10 ml), being not enough to dissolve the reactants in the process of UiO-66-NH2 creation. In contrast, with a large volume of solvent (≥22 ml), the crystallization of UiO-66-NH2 decreased due to the low concentration of reactants.
Figure 1. XRD patterns of (a) the UiO-66-NH2 synthesized in different reaction solvent volumes with reaction time of 24 h and (b) the UiO-66-NH2 synthesized in different reaction times with 16 ml of reaction solvent. Reaction temperature: 393 K; solvent exchange: CH3OH.
Download figure:
Standard image High-resolution image3.1.2. Effect of reaction times
The XRD pattern of UiO-66-NH2, synthesized in different reaction times (figure 1(b)), contained characteristic peaks of UiO-66 at 2θ = 7.34°, 8.48° [14–17] with different intensity dependences on the reaction times. The variation of peak intensity has extreme character. The intensity of characteristic peaks increased with increasing reaction times from 12 to 36 h, reached maximum at 36 h, then, when reaction time was increased up to 48 h, the characteristic peaks of UiO-66-NH2 decreased. This might be due to the short time (24 h) in which the reaction has not reached equilibrium, leading to low intensity peaks. When reaction time is 36 h, the reaction reach equilibrium, the intensity of peak became strongest.
3.1.3. Effect of exchange solvents
The experimental results showed that for the UiO-66-NH2 prepared in optimal conditions (16 ml of reaction solvent, temperature and time of reaction: 393 K in 36 h) and using CH2Cl2 as exchanged solvent, the highest specific surface area was obtained (SBET = 754.2 m2 g−1). Using C2H5OH or CH3OH as an exchanged solvent instead of CH2Cl2, the specific surface area of the sample decreased, reached SBET = 122.9 m2 g−1 and SBET = 576.4 m2 g−1, respectively. The boiling point solvent of CH2Cl2 (boiling point = 313 K) is lower than that of CH3OH (boiling point = 337.7 K) and C2H5OH (boiling point = 351.4 K), allowing the activation to be performed at lower temperature. Besides, CH2Cl2 has low acidity so its bonding with NH2 group is also weaker than that of CH3OH and C2H5OH, therefore, it is easier to separate during activated process.
3.1.4. Effect of activation time.
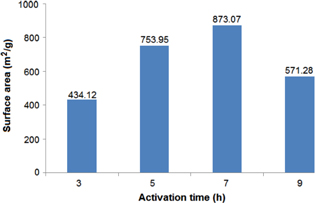
Generally, basing on results showed in figure 2, the specific surface area of product increased with activation time, and then decreased after reaching its maximum value. At the temperature 473 K, the activation times increased from 3 h to 5 h and 7 h, the specific surface area increased from 434.12 m2 g−1 up to 753.95 m2 g−1 and 873.07 m2 g−1, respectively. With further increasing the activated time to 9 h, the specific surface area decreased to 571.28 m2 g−1.
Figure 2. Surface areas of UiO-66-NH2 obtained at activated temperature 473 K.
Download figure:
Standard image High-resolution imageThe results of the above study allowed proposing the method for the preparation of UiO-66- NH2 as follows: the mixture including ZrCl4 (324 mg) and 2-aminoterephthalic axit (252 mg) was dissolved in 16 ml N,N-dimethylformamide (DMF). The solution was filled in a tightly capped vial, placed in a hot air oven and heated to 393 K overnight (36 h). The solid product then was removed by decanting with mother liquor and washed with DMF. After that solvent exchange was carried out with CH2Cl2. Finally, the solid product was activated under vacuum pressure at 473 K for 7 h. The physico-chemical characteristics of UiO-66-NH2 are introduced below.
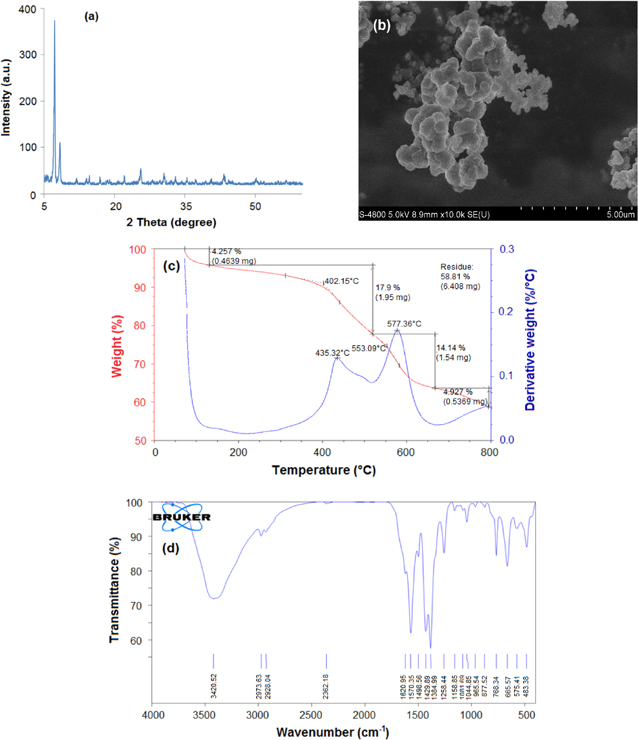
The XRD pattern in figure 3(a) allowed us to confirmed that UiO-66-NH2 was synthesized successfully with characteristic peaks of UiO-66 at 2θ = 7.34°, 8.48°. Figure 3(a) displayed narrow lines and high crystallinity of the sample and showed good agreement with that reported in the literature [14–17]. The morphologies of the sample are shown in figure 3(b). As is seen, the crystals of UiO-66-NH2 formed ball shape and the particle size was about 200 nm. The thermal stability of UiO-66-NH2 was investigated by thermogravimetric analysis (TGA) as shown in figure 3(c). Three weight loss steps were observed. The first weight loss of 4.3 wt% occurred between 303 K and 403 K due to vaporization of water. The second step of weight loss was 5.8 wt% at 403–673 K due to dehydroxylation of OH− at 523 K and was completed at 573 K [18]. The third step of weight loss was 22 wt% above 673 K due to decomposition of material. The result indicated that UiO-66-NH2 was stable up to 673 K.
Figure 3. Characteristics of UiO-66-NH2 prepared at the optimal conditions: (a) XRD pattern, (b) SEM imagine, (c) TGA diagram and d) IR spectrum.
Download figure:
Standard image High-resolution imageThe IR spectrum of UiO-66-NH2 is shown in figure 3(d). Overall it looked similar to the spectrum reported by Abid et al [17]. The appearance of absorption band at 1570.35 cm−1 indicated the existence of the reaction of –COOH with Zr4+. The band of 1506.02 cm−1 is referred to C = C from aromatic. The peak appearing at 3420.52 cm−1 is NH2 on the organic linker.
Synthesized UiO-66-NH2 showed high surface area up to 873 m2 g−1, micropore volume of 0.379 cm3 g−1, pore diameter of 18.96 Å and stability up to 673 K. Meanwhile, UiO-66 prepared by us in a previous study [15] obtained BET surface area 1041 m2 g−1, micropore volume 0.913 cm3 g−1, pore diameter of 30 Å and thermal stability up to 696 K [15]. From these data it follows that the addition of NH2 to UiO-66 led to a decrease in surface area, pore volume, and pore diameter.
3.2. CO2 adsorption on UiO-66-NH2
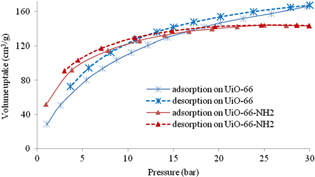
Figure 4 describes the CO2 adsorption and desorption isotherms of UiO-66 and UiO-66-NH2 at pressure range from 0 to 30 bar and temperature 303 K. At low pressure (≤15 bar), CO2 adsorption on UiO-66-NH2 is higher than that on UiO-66, although the surface area of UiO-66-NH2 is lower than that of UiO-66 (873 m2 g−1 compared to 1041 m2 g−1). This could be attributed to the presence of NH2 group in porous structure. At 1 bar and 303 K, CO2 adsorption on UiO-66-NH2 could reach 52.15 cm3 g−1, which is twice higher than that on UiO-66 (28.43 cm3 g−1).
Figure 4. Adsorption and desorption isotherms of CO2 on UiO-66 and UiO-66-NH2.
Download figure:
Standard image High-resolution imageIn contrast, at high pressure (15 to 30 bar), CO2 adsorption on UiO-66-NH2 is always lower than that on UiO-66. At 30 bar and 303 K, CO2 adsorption on UiO-66-NH2 is lower than that on UiO-66, reaching 145 cm3 g−1 và 167.7 cm3 g−1, respectively. The obtained result indicates that the presence of NH2 functional group increased the adsorption only at low pressure. The explanation for this result is that at low pressure, the adsorption capacity of CO2 of UiO-66-NH2 is high because of the effect of Lewis base center NH2, while the effect of specific area is still not clear yet. But at high pressure specific surface becomes the deciding factor for CO2 adsorption. Thus, in this condition, UiO-66-NH2 with lower specific surface area compared to UiO-66, expressed the lower adsorption capacity. Besides, the isothermal adsorption curve of CO2 on UiO-66-NH2 reaches saturated state at 30 bar. Meanwhile, the isothermal adsorption curve of CO2 on UiO-66 is continuously increasing at 30 bar. The explanation for this is that the adsorption process is limited by NH2 groups on the surface of UiO-66-NH2 at high pressure.
3.3. CH4 adsorption on UiO-66-NH2
The adsorption and desorption isotherms of CH4 on UiO-66 and UiO-66-NH2 at pressure 0 to 30 bar and 303 K are shown in figure 5. As seen, at range 0–30 bar and 303 K, CH4 adsorption on UiO-66-NH2 is always higher than that on UiO-66. At 1 bar and 303 K, the volumes of CH4 adsorption of 11.1 cm3 g−1 and 6.68 cm3 g−1 on UiO-66-NH2 and UiO-66, respectively, were obtained. At 30 bar and 303 K, CH4 adsorption on UiO-66-NH2 is also higher than that on UiO-66, reaching 57 cm3 g−1 and 49.1 cm3 g−1, respectively. The result indicated that the presence of NH2 groups is the more important factor affecting the adsorption ability of CH4 in comparison with specific surface area or pore volume. A similar result was reported by Wu et al [19].
Figure 5. Adsorption and desorption isotherms of CH4 on UiO-66 and UiO-66-NH2.
Download figure:
Standard image High-resolution image3.4. The adsorption of mixture 50%CO2/50%CH4
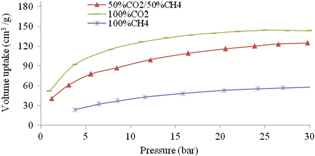
Figure 6 shows the increase of adsorption capacity of mixture CO2 and CH4 with the ratio 1:1 (v/v) on UiO-66-NH2 when pressure increases. At 30 bar and 303 K, on UiO-66-NH2 the CO2 adsorption is twice those of CH4, which were 145 cm3 g−1 and 75.38 cm3 g−1, respectively. It can be explained by the strong interaction between CO2 and adsorbent and high dipole moment of CO2 (13.4 × 10−40 C m2) while CH4 is nonpolar molecular [1]. The adsorption ability of the mixture of 50% CO2/50% CH4 is lower than that of pure CO2 but higher than that of pure CH4 on UiO-66-NH2, as shown in figure 6 also.
Figure 6. Storage volumes of gases on UiO-66-NH2.
Download figure:
Standard image High-resolution imageAdsorption characteristics of the mixture of 50%CO2/50%CH4 (figure 7) on UiO-66-NH2 and UiO-66 similar to CO2 adsorption (figure 5), i.e. at pressure lower 15 bar, the adsorption capacity of 50%CO2/50%CH4 mixture on UiO-66-NH2 was higher than that on UiO-66, but in the region of pressure higher than 15 bar, in contrast, the adsorption capacity of UiO-66 was higher than that of UiO-66-NH2. It can be related to the fact that CO2 storage capacity of the UiO-66 and UiO-66-NH2 is three times higher than that of CH4.
Figure 7. Storage volumes of gas mixture on UiO-66-NH2 and UiO-66.
Download figure:
Standard image High-resolution image3.5. Study on adsorption stability of UiO-66-NH2
The reusability of UiO-66-NH2 in adsorption process is also shown in table 1.
Table 1. Volume uptake of CO2 and CH4 on UiO-66-NH2 in ten adsorption-desorption cycles (from 1 to 10) under different pressures (P).
| Cycles of adsorption (cm3 g−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas | P(bar) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Standard error (%) |
| 1 | 52 | 51 | 55 | 50 | 42 | 53 | 52 | 53 | 50 | 52 | 1.1 | |
| CO2 | 14 | 133 | 136 | 132 | 132 | 138 | 134 | 135 | 137 | 137 | 132 | 0.78 |
| 30 | 145 | 148 | 142 | 144 | 155 | 145 | 147 | 149 | 150 | 143 | 1.25 | |
| 1 | 11.1 | 11.1 | 11.3 | 11.4 | 11.4 | 14 | 10.9 | 10.9 | 11.1 | 12.1 | 0.29 | |
| CH4 | 14 | 46.1 | 53.1 | 53.8 | 52.5 | 52.2 | 53.7 | 54.5 | 54.8 | 53.9 | 54.8 | 0.8 |
| 30 | 58 | 70 | 71.2 | 68.2 | 67.8 | 65.6 | 73.1 | 73.7 | 71.5 | 70.8 | 1.45 | |
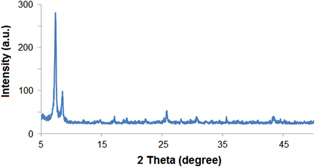
From the data of table 1 it follows that the adsorption capacity of CO2 and CH4 on UiO-66-NH2 are nearly unchanged after 10 cycles of adsorption–desorption. Besides, as was shown from the XRD spectrum (figure 8), the crystal structure of UiO-66-NH2 is nearly not changed after 10 adsorption–desorption cycles. These data confirmed that UiO-66-NH2 is a potential material for storage of gases even at high pressure. Besides, it could be easily regenerated by decreasing pressure at low temperature of desorption.
Figure 8. XRD spectrum of UiO-66-NH2 after 10 adsorption cycles.
Download figure:
Standard image High-resolution image4. Conclusions
UiO-66-NH2 was successfully synthesized; the optimal conditions for preparation were determined. The synthesized UiO-66 and UiO-66-NH2 should be considered as an effective adsorbent for CO2, CH4, and CO2/CH4 mixture. Unchanged structure of UiO-66-NH2 under high pressure adsorption process and reversible adsorption of CO2 and CH4 allow one to reuse UiO-66-NH2 many times.
The addition of amine groups to UiO-66 not only led to significant decrease in surface area, pore volume, and pore radius, but also increased its adsorption capacity; at 1 bar and 303 K, storage of CO2 and CH4 increased two-fold.
Comparing with UiO-66, addition of NH2 group enhanced the adsorption of CO2 and CO2/CH4 mixture at low pressure but showed an adverse effect at high pressure. Meanwhile, it increased the adsorption of CH4 even at high pressure.
Acknowledgments
The research group acknowledges the financial support for the program code VAST 03.01/13–14 from the Materials Science Council, Vietnam Academy of Science and Technology.