Abstract
Zinc oxide has emerged as a material of great interest due to its unique optical, electrical and magnetic properties. This review comprehensively covers the various aspects of zinc oxide tetrapods. Tetrapod is a one dimensional zinc oxide nano-microstructure and has been found to have very promising applications in diverse fields. The growth model, properties, synthesis methods and variations in the tetrapod morphology by varying the synthesis conditions have been discussed. The promising applications of zinc oxide tetrapod morphology have been also discussed in detail.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Zinc oxide and its nanostructures have gained remarkable interest since the last decade due to its unique combination of properties. The wide and direct bandgap (3.37 eV at room temperature), high exciton binding energy (60 meV), strong luminescence and large piezoelectric constants are only a few of the properties which make ZnO an exciting material for new technological functionalities [1]. ZnO can be fabricated in various nanostructured morphology like nanorods, nanowires, nanobelts, nanotetrapods (figure 1) and many more which bring about extraordinary changes in the properties of the material [2–4] Nanostructured ZnO materials have become a significant area of investigation because of their improved performance in the field of optics, electronics and photonics which pave the way for diverse nanotechnological functions. Reduction in size to a nano scale level introduces some novel electrical, optical and chemical properties which are mainly attributed to the surface and quantum confinement effects [5].
Figure 1. Scanning electron microscope (SEM) images of (a) 3D network of interconnected ZnO tetrapods [3] and (b) single ZnO tetrapod [4].
Download figure:
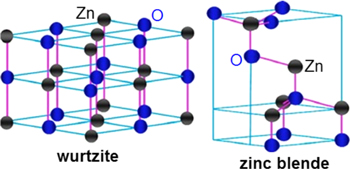
Standard image High-resolution imageZnO exhibits polytypism, i.e. it has more than one stable crystal structure (figure 2). It can exist both in the hexagonal wurtzite and cubic zinc blende structure [6, 7]. This behavior of ZnO allows for the formation of tetrapod like morphology where the four hexagonal wurtzite arms branch out from a central zinc blende core [8]. The (0 0 0 1) facets of the wurtzite structure and the (1 1 1) facets of the zinc blende structure are identical and these form the common intermediate facets between the two crystal structures and result into a tetrapod morphology [8]. However the formation of this structure requires the stability of these two crystal structures to reverse during the nucleation and growth process so that zinc blende structure formation takes place during the nucleation but wurtzite structure predominates during the growth process.
Figure 2. A schematic representation of ZnO crystal structures: wurtzite and zinc blende. The Zn and O atoms are marked with ash and blue circles respectively [7].
Download figure:
Standard image High-resolution imageTetrapod-shaped ZnO (T-ZnO) has several benefits over other nanostructured ZnO materials because of the spatial distribution of the legs of the tetrapod. One of the advantages of tetrapod over other nanostructured ZnO materials is that it gets automatically oriented on the substrate with one of its arms directed normal to the substrate surface [5]. ZnO tetrapods has an edge over the other ZnO nano-morphologies or its bulk counterparts for use in photovoltaic devices because tetrapod-shaped ZnO ensure better electron extraction when compared with other morphologies [9, 10].
2. Growth model of the tetrapods
The growth of the tetrapod-shaped ZnO is still not very clear though several attempts have been made to explain the growth mechanism. Most of the existing growth models for tetrapod-shaped ZnO focus on the existence of a seed nuclei from which the arms of the tetrapod grow out. These models differ in their interpretation of the geometry and crystallography of the nucleus [11, 12]. A growth model proposed by Shiojiri and Kaito suggests that the nucleus of the tetrapod-shaped ZnO has a zinc blende structure which has a crystallographic polarity along the 〈1 1 1〉 directions [13]. It has the shape of an octahedron where four of its eight surfaces are zinc terminated and the remaining four are oxygen terminated. The zinc terminated surfaces have higher surface energy and hence the growth is faster in a direction normal to the zinc terminated {1 1 1} surfaces resulting in a tetrapod structure. The four wurtzite crystal structured arms are assumed to grow on the zinc blende structured nucleus due to the stacking faults. The claim for a zinc blende type nucleus is based on an ambiguous diffraction pattern obtained after etching with HNO3 gas although there is no direct observational evidence for it. The existence of such a nucleus is ambiguous and even if it was formed then it should have transformed to wurtzite ZnO because cubic ZnO is unstable under ambient conditions. Also the angles between all the legs of the tetrapods from this model comes out to be 109.5° which does not agree with the optical spectroscopy measurements made by Fujii et al [14] and electron microscopy measurements by Abe et al [15].
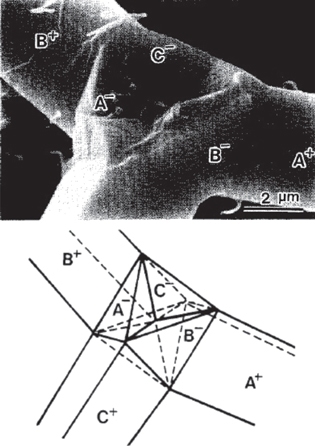
A growth model known as the octa-twin model was proposed by Iwanga et al [16]. This model was based on the measurements of angles between the legs of tetrapod-shaped ZnO. The growth was described to occur in three steps. Firstly, an octahedral multiple twin nucleus consisting of eight trigonal pyramid crystals is formed. Such a geometry does not result in the formation of a perfect octahedron since the angles between the planes is 85.5° and not 90°. Hence the second step is to relax the strain energy resulting from the large misfit and this is done through a decohesion or cracking at the twin boundaries (figure 3). The third step involves the preferential growth of the legs from four of the crystals in the octa-twins in the +c direction resulting in a tetrahedral morphology. However a major drawback of this model is that no direct evidence exists for the existence of octa-twin arrangement though certain evidence has been provided by authors based on the transmission electron microscopy observations [17].
Figure 3. Scanning electron micrograph of a fractured tetrapod along with its possible crystal configuration as per the octa-twin model [17].
Download figure:
Standard image High-resolution imageNishio et al proposed a model addressing the issues in the earlier growth models [18]. It was stated that a zinc blende type nucleus is formed in early stages under the special conditions of high pressures and temperatures which eventually phase transforms to a more stable wurtzite-ZnO by a mechanism of slipping of lattice planes. This mechanism generates multiple-twinned nuclei having 4–8 elements which promotes further growth of ZnO tetrapods. Also the angles between the arms of the tetrapods are in quite a good agreement with earlier experimentally obtained values [14]. A more recent study by Ronnig et al suggests that the nucleus consists of four grains of hexagonal shape having a twin like relation which form a distorted tetrahedral configuration to accommodate the strain arising out of the misfit between the grains [11]. Such a nucleus is said to be energetically favorable as it has the minimum ratio between the grain boundary areas and the surface areas compared with other multiple grain geometries. The four grains grow preferentially in the +c direction and form the four legs of the tetrapod-shaped ZnO. However the growth mechanism of the ZnO tetrapods is still not fully understood and remains an area to be investigated.
3. Synthesis of tetrapod-shaped ZnO
3.1. Chemical vapor transport and condensation
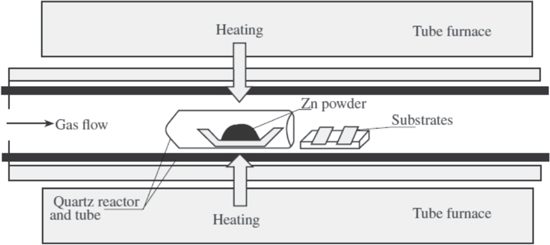
This is the most common technique used for the synthesis of ZnO tetrapods [19]. In this method, zinc and oxygen vapor react with each other forming various ZnO nanostructures [20]. However, synthesis of a particular kind of nanostructure such as tetrapod-shaped ZnO requires a careful control of the reaction parameters. There are several ways to generate the zinc and oxygen vapor classified as direct and indirect methods. The direct method involves the heating up of zinc powder under controlled oxygen flow (figure 4) [21–26]. Lower temperatures are required for synthesis of tetrapod-shaped ZnO nanostructures by this method but the zinc to oxygen vapor ratio must be maintained carefully as a change in this ratio brings about a change in the morphology of the product [22]. Shiojiri et al fabricated the ZnO tetrapods by burning of zinc powder in a gas mixture comprising of 20% oxygen and 80% argon [13, 27]. Tetrapod-shaped ZnO was formed by oxidation of the evaporated zinc vapor in the gas phase.
Figure 4. Schematic of the chemical vapor transport and condensation (CVTC) process [25].
Download figure:
Standard image High-resolution imageFor the indirect method synthesis (equations (1) and (2)) a mixture of zinc carbonate powder (ZnCO3 · 2Zn(OH)2 · H2O) and graphite powder is used as the source of zinc vapor [19, 20, 28–33]. This synthesis is usually carried out in a horizontal tube quartz furnace where the source material for the reaction is placed in a crucible in the central zone of the furnace and the furnace is heated to a temperature of 900 °C. Argon gas with 0.5 to 5% oxygen content is made to flow in the furnace and in the downstream of gas flow Si substrates are placed to collect the fabricated tetrapod-shaped ZnO. The mechanism for this is the vapor phase nucleation and growth of the tetrapods. Initially the carbothermal reduction of ZnCO3 takes place at elevated temperatures [5, 34]. With increase in temperature the ZnCO3 is converted to ZnO as per the reaction:
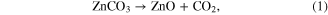
The graphite then deoxidizes the ZnO to various suboxides form:
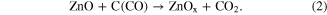
The supersaturated zinc is then carried to the oxygen containing gas where nucleation and growth of the tetrapod-shaped ZnO takes place as per the growth mechanisms described earlier in this paper.
3.2. Flame transport synthesis
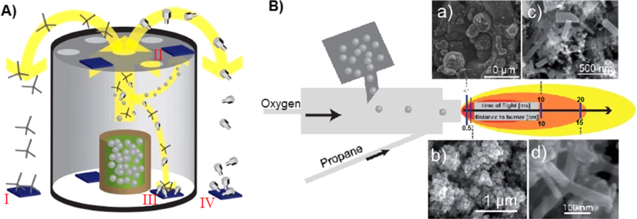
This method has been recently developed by Mishra et al with four variants of this approach [35, 36]. The first variant does not yield ZnO tetrapods but results in the formation of nanoseaurchin shaped ZnO. For the first variant a slurry of Zn powder, polyvinyl butyral (PVB) and ethanol is made with the constituents in appropriate ratio and a coating of this is applied on the substrate. Subsequently the substrate is dried and heated to form nanoseaurchin type structures. For the second variant, a mixture of Zn powder and PVB is taken in the ratio of 1:2 in a ceramic crucible and heated to a temperature of 900 °C for 30 min. During heating of the PVB, it undergoes combustion and the zinc microparticle powder transforms to the building blocks of the nano-micro tetrapod morphology which are simultaneously carried by the flame (figure 5(a)). The yield from this approach was about 30–40%. The third variant utilizes a slurry same as used in the first variant but in this variant we place the slurry in a crucible and use a special type of ceramic cylindrical arrangement through which a variety of ZnO nano-microstructures can be synthesized simultaneously by placing the substrates at different positions inside or outside of the ceramic cylinder. Here the synthesis temperature ranges from 800–1050 °C. A fourth variant involves the use of a burner with three inlets (figure 5(b)). Oxygen and propane gas are introduced from two of the inlets and zinc powder is inserted from the third inlet. Various morphologies of ZnO can be synthesized by placing the substrate at appropriate distance from the burner. Interconnected 3D networks of tetrapods with high porosity have also been fabricated by compressing the tetrapod powder in a ceramic tube followed by subsequent annealing to a temperature of 1150 °C.
Figure 5. (A) Schematic for controlled variant of flame transport synthesis (I–IV). Substrate positions I, II, and IV lead to growth of selected type structures, however, the substrates at position III will have a mixture of all kinds of structures depending on their locations. (B) Burner approach of flame transport synthesis, (a-d) different structures formed at different distances from the flame [35].
Download figure:
Standard image High-resolution image4. Controlling the morphogenesis of tetrapod-shaped ZnO
The chemical vapor transport and condensation (CVTC) process was used for the synthesis and achieved control over size and shape of the tetrapods by simply varying the growth times, reaction temperature and partial pressure of oxygen by Yan et al [37]. Size was controlled by varying the growth times and the ratio of zinc vapor pressure to the partial pressure of oxygen. Shape was controlled by varying the ratio of the zinc vapor pressure to partial pressure of oxygen. An argon-oxygen mixture consisting of 0.5–5% O2 resulted in uniform ZnO tetrapods with hexagonal arms with a significantly high yield of 95%. Tetrapods having trumpet like arms (figure 6) were reported to grow when the O2 partial pressure was greater than 5%. When the O2 partial pressure was increased in the range from 0.5 to 5% an increase in the diameter of the tetrapods was reported from 100 nm to 2 μm. The thicker tetrapods have uniform hexagonal cylindrical arms while the thinner ones have needle shaped arms.
Figure 6. Scanning electron microscope images of trumpet shaped ZnO at (a) low and (b) high magnification [37].
Download figure:
Standard image High-resolution imageZhang et al [38] also used a chemical vapor transport and condensation (CVTC) growth process but report that the flow rate of oxygen and carrier gas has a negligible influence on the morphology of the tetrapods. Instead of this condensation temperature and the substrate cooling procedure are said to play a more vital role in the morphology control. Bhomanee et al [39] fabricated the tetrapod-shaped ZnO by thermal oxidation of zinc powder with different solutions of methanol, ethanol and H2O2. The smallest size and the best morphology is reported to grow by the use of hydrogen peroxide solution. Also the yield was maximum for the same solution. The role of H2O2 was to act as a strong oxidizer and supply oxygen to zinc to form the ZnO tetrapods.
Vanithakumari et al [40] used a thermal evaporation technique of the zinc granules without the use of a catalyst or carrier gas flow. The effect of temperature gradient in the furnace along with the effects of vaporization temperature of zinc and the growth temperature has been studied. It was found that the vaporization and growth temperature have opposite impact on the aspect ratio of the tetrapods. A higher vaporization temp of zinc favors the fabrication of longer and thinner tetrapods whereas higher growth temperatures result in thicker tetrapods with larger diameters. A zero temperature gradient such as that present in the central zone of the furnace (dT/dx = 0 since Tmax occurs here) leads to tetrapods having legs of uniform diameter whereas the tetrapods collected from zones having a high temperature gradient such as that present between a low and high temperature zone, result in the formation of tetrapods having uniform leg diameter up to the middle followed by a decrease in the diameter thus forming a sharp tip.
5. Properties of tetrapod-shaped ZnO
5.1. Optical properties
The optical properties of ZnO nanostructures have been reviewed in a great detail by Sirbuly et al [41]. Several ZnO nanostructures exhibit the property of optically pumped lasing and Huang et al was the first to demonstrate this phenomena in ZnO nanorods [42, 43]. Photoluminescence (PL) measurements have been performed by many research groups [44–46]. The PL measurements indicate the existence of two emission peaks corresponding to ultraviolet (UV) emission at 380 nm occurring at around 3.2 eV and green emission at 495 nm occurring at 2.3 eV. The UV emission relates to a near band-edge emission caused by interband transitions and the green emission has been proved by Vanheusden et al and Wang et al [47, 48] to be an outcome of singly ionized oxygen vacancies. Photoemission results from the recombination of a photogenerated hole with the electron occupying the oxygen vacancy site. The UV emission for the bulk ZnO is detectable only at very low temperature but for the tetrapod-shaped ZnO it was detectable even at room temperature owing to mainly two reasons—first being the high quality and lesser impurities and structural defects in ZnO tetrapods and the other reason is related to the quantum confinement effect in nanostructures. Newton et al have observed quite a remarkable difference in response from a group of randomly oriented tetrapods and a single tetrapod (figure 7) oriented in a manner such that one of its arm is aligned parallel to the incident laser beam [49–51]. This difference in behavior is reasoned on the basis that the vertical leg of the aligned tetrapod behaves like a Fabry–Perot resonant cavity [52].
Figure 7. Room temperature photoluminescence of a ZnO tetrapod cluster and an isolated tetrapod [5].
Download figure:
Standard image High-resolution image5.2. Electronic properties
The difficulty in making electrical contacts to the nanocrystals has limited the study of their electronic properties. Newton et al have fabricated a diode structure with a single tetrapod and electrical contacts to the tetrapod have been made by metallo-organic deposition assisted by focused ion beam (FIB) technology [5, 53]. Three electrical contacts were made to the tetrapod-two were W contacts which are ohmic in nature and one is a Pt contact which behaves as a Schottky contact. The rectification behavior of such an arrangement was studied. Also the electrical response of the ZnO tetrapod Schottky diode was observed and using a continuous wave laser illumination and it was found that with a increase in the laser power density, the behavior of this device shifts from a rectifying one towards a more ohmic side. Heo et al [54] had obtained similar results on performing the experiments with a single nanorods Schottky diode. Wan et al [55] synthesized the tetrapod-shaped ZnO by direct thermal evaporation and investigated it for field emission. A very low turn on field of 1.6 V μm−1 at a current density of 1 μA cm−2 is reported due to the high aspect ratio of the ZnO tetrapods.
6. Applications of tetrapod-shaped ZnO
The applications of ZnO tetrapods are multidimensional. Its unique combination of properties along with a special structural morphology allows it to be used for a vast variety of applications which are still being explored. A recent study by Xin et al [4] makes uses of the tetrapods for the development of self-reporting materials based on optical response. The self-reporting behavior of these materials is based on the photoluminescence of ZnO tetrapods which varies with stress. A tetrapod-shaped ZnO (T-ZnO)/polydimethylsiloxane (PDMS) elastomeric composite was fabricated and the special feature of this composite was that unlike the randomly distributed short fillers which align in the direction of stress and have no restraining effect, the tetrapods always have legs which are not parallel to the direction of applied stress and hence have a restraining effect by interlocking mechanism. As the stress is applied, the elastic energy first gets stored in the T-ZnO network thus stiffening the composite and when the critical stress is reached this network breaks and the energy is released. The observed optical response is due to the defects generated by stress during the bending and rupturing of the tetrapods. This phenomenon allows for the non-destructive evaluation of the internal damage in the material and hence opens up new avenues for its utilization in diverse engineering applications.
A novel application of the tetrapod-shaped ZnO was recently published by Xin et al on joining of the low surface energy polymers by using T-ZnO as the linker [56]. In this study an extremely difficult to join combination of polytetrafluoroethylene (PTFE) and polydimethylsiloxane (PDMS) were joined and significantly high peeling strengths are reported. The concave tetrapods act as micro/nano Velcro filler at the interface of the polymers unlike the convex shorter fibers. This leads to a very good adhesion between the polymer layers simply on the basis of mechanical effects without any chemical interaction. The geometry of the tetrapods is such that if it is tried to pull out then it causes a significant bending of its legs or deformation of the polymer in many embedding situations. This method of joining of materials can be complemented with other joining methods such as plasma methods to increase the magnitude of adhesive force between the materials and are especially important in cases when the materials cannot be joined by the conventional methods.
Mishra et al [57, 58] have investigated the antiviral activity of the tetrapod-shaped ZnO against the HSV-1 (Herpes simplex virus-1) and HSV-2 (Herpes simplex virus-2) viruses and report that tetrapod-shaped ZnO can significantly reduce the entry of these viruses into the cell by mechanism of their attachment to the surface of ZnO nanostructures. The inherent oxygen vacancies cause the virus to attach to the surface of the tetrapod arms. Since oxygen vacancies are responsible for the attachment of the viruses, the efficiency can be increased by illumination under UV light which increases the concentration of oxygen vacancies in these structures. Also the cytotoxicity of the ZnO tetrapods is very low as compared to other nanostructures which make it an attractive future material for biomedical engineering.
Mecklenberg et al [59] made use of T-ZnO templates to fabricate a novel light weight material named aerographite which is one of the least dense materials in the world. The ZnO templates were prepared by the second variant of flame transport synthesis approach followed by compression of the tetrapod powder and subsequent annealing to form the interconnections. Chemical vapor deposition (CVD) is performed on this templates using toluene as the precursor under appropriate gas flows. The template was finally removed by reducing ZnO to metallic zinc by H2 reduction which is then precipitated out through the exhaust system. This material has far reaching applications in areas where electrically conductive, porous and mechanically stable structures are a requirement like in Li-ion batteries or supercapacitors.
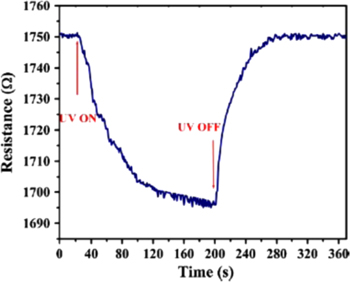
The T-ZnO has also been successfully applied for sensors. Lupan et al [60] used an in-situ lift out technique for ZnO tetrapods fabricated in an aqueous solution to prepare a single ZnO tetrapod UV sensor. This sensor showed a decrease in the resistance (figure 8) when it was illuminated with UV light. The decrease in resistance of the T-ZnO sensor is attributed to desorption of the chemiadsorbed oxygen from the surface and increase in charge carrier density due to electron-hole pair generation. Gedamu et al [61] fabricated an interpenetrated network of T-ZnO in a gap created by fracture of thin film of Au. The network of ZnO tetrapods was fabricated by using the fourth variant of the flame transport synthesis (FTS) approach and 1D networks of ZnO were also fabricated through the third variant of FTS for the comparison of these sensors. These fabrication approaches allowed a direct integration of the nanostructures in the gap for bridging the electrical contacts on chips. The UV sensor made by fourth variant of FTS i.e. the burner approach, exhibited faster recovery and response times along with a high signal ratio. UV sensors have also been fabricated by other groups like Rackauskas et al [62] who dried a drop of T-ZnO solution between two single walled carbon nanotube (CNT) as the electrical contacts on a polyethylene terephthalate (PET) substrate.
Figure 8. The resistance change of the ZnO tetrapod-based sensor under UV irradiation [60].
Download figure:
Standard image High-resolution imageCarotta et al [63] have investigated the relative gas sensing efficiency of ZnO sensors fabricated through two routes, one fabricated by sol-gel route and the other are tetrapods fabricated by a vapor transport and oxidation process. They found out that that the gas sensing ability of both these sensors were roughly equal for the gases tested i.e. O3, NO2, CO and H2S but the sensing response for CO was much better for sol-gel sensors than tetrapod sensors. Also enhanced response is reported for oxidizing gases particularly ozone and the sensors were found to be effective for detecting even ppb levels of ozone. Delaunay et al fabricated a T-ZnO gas sensor for ethanol which showed an improved performance over other ZnO sensors with a factor as large as 20 at an ethanol concentration of 100 ppm [64]. ZnO tetrapods have a bright prospect as a sensor material due to a highly porous network permitting a high surface to volume ratio for gas sensing.
Silica-coated amino-modified ZnO tetrapods were fabricated by Nie et al [65] and were used as novel carriers for plasmid DNA delivery. The tetrapods with their unique morphology bind to the cell membranes with their three legs attached to the cell for DNA delivery but their complex morphology does not permit them to pass through the cell membrane. Similar to phages which deliver genes to cells without entering them, T-ZnO also deliver plasmid DNA into the cells without entering them and which in turn decreases any cytotoxic effects. The photocatalytic activity of ZnO tetrapods is also being studied in detail as it has future potential for applications like waste remediation and water purification. T-ZnO's distinct three dimensional branched morphology prevents it from aggregating in solution, thus providing a larger effective surface area over other nanostructures for the photocatalysis to take place [66].
The field emission properties of the T-ZnO by coating it on a Nickel and doped-Silicon substrates was investigated by Wan et al [55, 67]. For the former, a low turn on field of 1.6 V μm−1 has been observed at a current density of 1 μA cm−2. Also very low degradation rate of the cathode has been reported which make ZnO tetrapod an ideal material for cold cathodes and vacuum electron devices in field emission displays. Jun et al have synthesized ZnO-lead-zinc-borosilicate glass varistors using T-ZnO which required lower sintering temperatures for varistor fabrication because of the higher activity of the ZnO tetrapod powder and demonstrated a high non-linear coefficient α of 35 along with leakage current lesser than 5 μA [68]. Dye-sensitized solar cells using T-ZnO has been demonstrated by Hsu et al [69]. Efficiency of 1.2% has been reported and hence T-ZnO can prove to be a promising material in this field with further developments. A significant amount of research is in progress on doping ZnO with Mn or Co for creating a dilute magnetic semiconductor (DMS) [70, 71].
7. Conclusion
This review has comprehensively covered all the major aspects of the zinc oxide tetrapods including its synthesis, growth model, properties, morphology control and applications. The flexibility and high-temperature stability of these conducting and highly porous networks formed from these tetrapods can be used for various technological applications. Considering its structural morphology coupled with the unique combination of properties for nanostructured ZnO materials, T-ZnO appears to be a promising material for future technological applications in diverse fields but there is still a lot of room for research for studying the evolution of the tetrapod morphology and to further explore the new horizons of its applications.
Acknowledgments
The author certifies that he has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.