Abstract
Zinc oxide (ZnO) nanoplates and tungsten trioxide (WO3) nanorods were synthesized by hydrothermal treatment from zinc nitrate/potassium hydroxide and sodium tungstate/hydrochloric acid, respectively. The structure, morphology and compositions of the as-prepared WO3/ZnO nano-composites were characterized by x-ray diffraction, field emission scanning electron microscopy and energy dispersive spectroscopy. The obtained ZnO nanoplates have regular shape, single-crystal wurtzite structure with the thickness of 40 nm and 200 versus 400 nm in lateral dimensions. The WO3 nanorods possess the average diameter of 20 nm and the length of approximately 120 nm which were distributed on the surfaces of ZnO nanoplates. The WO3/ZnO nano-composites were prepared by grinding WO3 nanorods powder with ZnO nanoplates powder in various weight ratios (1:2, 1:1 and 2:1). The NH3 gas sensing properties of WO3/ZnO nano-composites were examined through the electrical resistance measurement. The gas sensing performance of the WO3/ZnO composite with weight ratio of 1:1 was better compared with that of other samples. For this sample, the maximum response to 300 ppm NH3 was 24 at the operating temperature of 250 °C. In addition, the gas sensing mechanism of the WO3/ZnO composites was discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Gas sensing performance of metal oxide semiconductor has been attracting considerable attention for a long time because of its great potential to solve environment problems [1, 2]. Oxide nanomaterials (such as WO3, ZnO, CuO, Fe2O3...) possess novel optical and electrical properties and have many applications in nanomedicine [3], nanosensors [4], catalyst [5], optical and optoelectronic devices [6]. Among the semiconductors employed, zinc oxide (ZnO, a typical kind of II–VI compound semiconductor) is an important metal oxide with the wide direct band gap of 3.37 eV [7]. Nanostructures of ZnO are the most widely studied material because of its chemical and physical characteristics. On the other hand, WO3 is an attractive material which shows gas sensitivities covering a wide range of concentrations and fast response as well as good selectivity [8]. In most of the gas sensing applications, large surface area is required for diffusion and interaction of reactive species with active sites.
In principle, the coupling of different semiconductor oxides can reduce its band gap, promote electron–hole pair separation and consequently achieves a higher gas sensing activity. We expect that the potential difference across such a heterojunction can increase the effective energy barrier and thus enhance the gas sensing performance and shorten the response. The structure of WO3/ZnO nano-composite for gas sensors consists of an interfacial metal oxide layer to increase gas sensitivity and provide thermal dynamic stability over their electrical characteristics, so the sensing performance of such sensors rely on the barrier height variation [9]. In the past years, coupled semiconductors were formed by ZnO and other metal oxides such as CuO for selective H2S detection [10] or WO3 for H2 gas sensing [11] and for photocatalytic degradation [12]. In this contribution, we prepared WO3/ZnO composite by mixing WO3 nanorods (NRs) powder and ZnO nanoplates (NPls) powder which had been synthesized separately by hydrothermal treatment to accomplish uniform nanoscale WO3–ZnO junctions and achieve rapid and effective diffusion of target gases to the sensor surfaces. We studied the ammonia sensing properties of the WO3/ZnO nanocomposite and experimental results exhibit better performance, faster response and higher sensitivity than those of pure WO3 or pure ZnO based ammonia sensors.
2. Experimental
For preparing ZnO NPls, zinc nitrate hexahydrate Zn(NO3)2 · 6H2O (99%, China) and potassium hydroxide (KOH 85%, China), absolute ethanol (99.6%, China) were used as precursors. All the reagents used were analytical grade. First, 19.8 g KOH powder was dissolved in 200 ml distilled water and stirred for 5 min to obtain 1.5 M KOH solution and 7.512 g Zn(NO3)2 · 6H2O was dissolved in 50 ml distilled water to obtain 0.5 M Zn(NO3)2 solution. Then, 83 ml 1.5 M KOH solution was slowly added into 0.5 M Zn(NO3)2 solution (50 ml) with molar ratio of 2:1 and continuously stirring for 15 min. The reaction between KOH and Zn(NO3)2 solutions results in a white precipitate which was transferred to a teflon-lined stainless steel autoclave which was then sealed and heated at 180 °C for 20 h. After the hydrothermal treatment, the product was collected by filtration and repeatedly rinsed with distilled water and ethanol several times. The last product was dried in air in laboratory oven at 80 °C for 24 h to convert the precursor to pure ZnO.
WO3 NRs were synthesized by hydrothermal treatment using sodium tungstate dihydrate powder (Na2WO4 · 2H2O, England), hydrochloric acid (HCl, China), and absolute ethanol (C2H5OH, China). First, 4.125 g Na2WO4 · 2H2O was dissolved in 12.5 ml distilled water and stirred continuously for 30 min to form a translucent, homogeneous solution. Then, HCl solution (3 M) was dropped into the Na2WO4 solution so that the pH value of the solution varied from 1.8 to 2.2 during stirring process. After stirring at room temperature for 4 h, the obtained solution had a white–yellow color. The system was then transferred to a 20 ml teflon-lined autoclave and subjected to hydrothermal treatment at 120 °C for 24 h. Finally, white glue precipitates were collected and washed with absolute ethanol and distilled water several times to remove Na+, Cl− ions in the products, and dried in air at 80 °C for 24 h.
In order to prepare WO3/ZnO composite, 0.04 g WO3 NRs powder and 0.04 g ZnO NPls powder were weighed and dispersed in 0.4 ml distilled water, respectively. Then, pure WO3 NRs and pure ZnO NPls solutions were mixed in WO3/ZnO weight ratios of 2:1, 1:1 and 1:2 followed by a grinding process.
The structure property of the WO3/ZnO nanocomposite was determined by x-ray diffraction (XRD, Bruker D8 Advance x-ray diffractometer, Germany) with Cu-Kα radiation (λ = 1.5406 Å) and scan rate 0.03° s−1, scanning angle from 20° to 70°. The material morphology was observed by a scanning electron microscope (SEM, Hitachi S4800 Japan) operating at 10 kV. Elemental composition of samples was determined by energy dispersive x-ray spectroscopy (EDS, using OXFORD JEOL 5410 LV, Japan).
For the gas sensing measurement, the slurry solution was drop-coated onto a Pt-interdigitated electrodes (the electrode gap is 20 μm) and then dried at 80 °C for 24 h. Subsequently, the sample was annealed at 400 °C for 2 h in air to stabilize the oxide layer and decrease its defects. The performance of the sensor was examined by a static gas testing system.
3. Results and discussion
The surface morphology of the WO3 NRs and ZnO NPls were showed in figure 1. The pure WO3 materials exhibit well-defined NRs with the average diameter of ∼20 nm and the length of 120 nm. Figure 1(b) showed ZnO NPls with the average thickness of about 40 nm and the average size of 200 × 400 nm. There was no considerable difference in the plate dimension. The existence of the secondary oxide (WO3) made the surface of ZnO NPls rougher (figure 1(c)).
Figure 1. SEM images of (a) WO3 NRs, (b) ZnO NPls and (c) WO3/ZnO composite.
Download figure:
Standard image High-resolution imageFigure 2(a) shows XRD pattern of WO3 NRs sample, the diffraction peaks can be indexed to hexagonal structure WO3 (h-WO3) (JCPDS card number 75-2187) with lattice constants of a = b = 0.7298 nm, c = 0.3899 nm, α = β = 90°, γ = 120°, space group of primitive cell is P6/mmm [13]. No characteristic peaks from other impurities are detected, demonstrating the high purity of the products. Figure 2(b) shows XRD pattern of pure ZnO NPls sample obtained by wet chemical technique, the strong diffraction peaks in the pattern are indexed to the hexagonal wurtzite structure with lattice constants in accordance with values in the standard card (JCPDS card number 79-0205 for ZnO, a = b = 0.3242 nm, c = 0.5188 nm, α = β = 90°, γ = 120°, space group P6/3mc) [14]. The narrow peaks indicate that the material has a good crystallinity. As estimated from the half-peak width by Debye–Scherrer's equation [14], the average crystallite sizes of WO3 and ZnO were about 20 nm and 42 nm, respectively. Figure 2(c) indicated that the WO3 NRs are well mixed in the ZnO matrix.
Figure 2. XRD patterns of (a) pure WO3 NRs, (b) pure ZnO NPls and (c) WO3/ZnO composite.
Download figure:
Standard image High-resolution imageThe formation of WO3/ZnO composite is also confirmed from the EDS analysis. EDS spectra (figure 3) show that three main elements Zn, W and O are existed in the samples. Semi-quantitative analysis of the weight concentration (wt%) reveals that estimation of the WO3/ZnO ratio in the sensing layer on the basis of EDS data was in good agreement with the designed concentrations. In some cases, element carbon (C) was also detected, which probably originated from the supporting carbon tape.
Figure 3. EDS of WO3/ZnO nanocomposites.
Download figure:
Standard image High-resolution imageThe response to NH3 concentration in air ranging from 25 ppm to 300 ppm was measured at various temperatures (200 °C–400 °C) upon the exposure and removal of NH3 source (figures 4(a)–(d)). The sensor showed a typical n-type sensing behavior, in which the resistance of the films abruptly decreases in the presence of NH3 and returns to the original value upon exposure to air. The response to 25 ppm NH3 at 250 °C of WO3:ZnO = 1:1 in weight composite sample was 9 (figure 4(b)), indicating that NH3 could be detected at ppm-level using WO3/ZnO sensor. For the pure ZnO NPls, the response to 300 ppm NH3 was from 1.6 to 3.6 corresponding with the temperature from 250 °C to 400 °C (figure 4(d)). In contrast, the response to 300 ppm NH3 of WO3:ZnO = 1:1 in weight composite samples was from 3 to 24, which was substantially higher than that of the ZnO sensor at all tested temperatures. The sensor response under exposure to 300 ppm NH3 was also recorded for the composite films at optimal operating temperatures and the results are introduced in figure 4(e). The pure ZnO NPls material exhibits rather low response compared with the composite nanomaterials.
Figure 4. Response characteristics of the WO3/ZnO composite sensors with different weight ratios depending on NH3 concentration at optimum working temperature (a)–(c), operating temperature (d), composition (e) and response and recovery times to 300 ppm NH3 (f).
Download figure:
Standard image High-resolution imageThe sensor response of all the samples to NH3 increases with the operating temperature and reaches maximum at 300 °C. Except for the WO3:ZnO = 1:1 composite, the response reaches the highest value at 250 °C. Therefore, the film derived from WO3:ZnO = 1:1 composite material shows the highest response to NH3.
As the concentration of ammonia increases, more NH3 will be adsorbed on the WO3/ZnO surface and a larger barrier height change was observed. After the ammonia concentration reaches 300 ppm, the NH3 absorption tends to be saturated.
The response and recovery times of the composite samples are shorter than those of pure WO3 sample, but longer than those of pure ZnO sample. Figure 4(f) showed the response time (τres = 60 s) and the recovery time (τrec = 50 s) for exposure to 300 ppm NH3 of sample 1:1 at 250 °C, whereas response time and recovery time of pure WO3 nanorods based-sensor at 50 °C were 10 min and 2 min [15], at 300 °C were 110 s and 40 s, and those of pure ZnO at 300 °C were 43 s and 34 s, respectively. Although the sensitivity and the optimal working temperature were improved significantly, slow rate of response and recovery was the main disadvantage of this samples (WO3:ZnO = 1:1 composite sensor).
Figure 5 shows the selectivity of the gas sensor based on WO3 NRs/ZnO NPls composite with composition of 1:1 in weight. The sensor was exposed to NH3, C2H5OH, CH3COCH3, LPG of the same concentration of 25 ppm at 250 °C. It can be seen that the response to NH3, C2H5OH, CH3COCH3, LPG are 10, 3, 8, 3, respectively. This sensor exhibits the highest response to NH3.
Figure 5. Response of WO3/ZnO = 1:1 composite sensor to 25 ppm NH3, ethanol (C2H5OH), acetone (CH3COCH3) and LPG at 250 °C.
Download figure:
Standard image High-resolution imageIn case of the WO3/ZnO heterojunction, Anderson model is used to explain the energy band diagram [16], with the assumption that the interface states can be neglected. Due to the similarity in crystal structure (hexagonal for both materials), it was expected that there would be negligible strain at the interface. The bandgaps of the two oxides are 2.7 eV and 3.4 eV, respectively [17]. The conduction and valence band edges are discontinuous at the junction where space–charge neutrality is assumed to exist at every point. So we can obtain the theoretical energy band diagram as shown in figure 6, which depicts the equilibrium condition for n–n hetero-junction and the Fermi level in WO3 coincided with that in ZnO. It is seen that there exist additional potential barriers at the boundary between WO3 NRs and ZnO NPls which could play role in improving the sensitivity of the sensor.
Figure 6. Energy band diagram of the WO3/ZnO hetero-junction under air ambience.
Download figure:
Standard image High-resolution imageWhen the WO3/ZnO surfaces are exposed to ammonia, the NH3 molecules diffuse through the metal oxide layer and the WO3/ZnO interface, then penetrate into the film. The NH3 molecules react with the negatively charged surface oxygen ions which are located at WO3/ZnO interface and their surfaces as following:
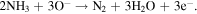
Electrons are released and lower the barrier height substantially, allowing more electrons to flow through the oxide layer. The conductivity increase by the oxidation of NH3 with the reaction of O− (or O2−) at the WO3/ZnO interface and surface is responsible for NH3 sensing property. The enhanced response to NH3 in the present study can be explained in part by the effective diffusion of target gas toward entire sensing surface. The WO3 NRs loading on ZnO NPls can affect gas sensing characteristics by creating potential barriers at the hetero-junctions, additional to well-known Schottky barriers existing at the surface of ZnO nanocrystals.
4. Conclusion
The WO3 NRs and ZnO NPls were synthesized by hydrothermal treatment and WO3/ZnO composites were prepared by mixing process in which the weight ratio were modulated of 2:1, 1:1 and 1:2. The optimum performance was obtained for the composite derived from WO3 NRs with 50% ZnO NPls in weight. The optimum operating temperature was from 300 °C to 250 °C and the sensitivity was improved from 3 (pure ZnO sample) to 24 (1:1 WO3/ZnO composite). The enhancement of the porosity and the existence of the hetero-junctions between WO3 and ZnO may lead to the better sensitivity upon exposure to NH3 in the WO3/ZnO composite sensor. It was hypothesized that the gas-sensing properties of WO3/ZnO nano-composites are determined not only by their specific surface area but also by their porous structure and electronic state of the hetero-junction. In summary, it is expected that WO3 NRs/ZnO NPls composite would be a potential candidate ammonia sensor.
Acknowledgments
This work is supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.99-2015.18.








