Abstract
In order to detect trace concentrations of organic or biological molecules by surface-enhanced Raman scattering (SERS) technique, the SERS-active substrates with high enhancement factor are required. The silver nanodendrites (AgNDs) are a growing class of such SERS-active substrates. This report presents the preliminary results of the trace detection of paraquat (PQ), a commonly used herbicide, with the use of SERS-active substrates, which have been made from AgNDs deposited on silicon. The AgNDs were produced either by electroless deposition, or by electrodeposition onto a silicon wafer, using aqueous solution of HF and AgNO3. It was observed that the silver dendrites are formed only when AgNO3 concentration is high enough. Next, it was found that with the additional assistance of an electric potential in the electrodeposition, the dendrites have grown up with the more perfect ramification. The AgNDs with more perfect branching gave the Raman spectrum of PQ with higher enhancement factor. More specifically, while the SERS-active substrates prepared from electrodeposited AgNDs were able to detect PQ with concentration as low as 0.01 ppm, the ones made from electroless deposited AgNDs could only detect PQ at concentration of hundreds times higher.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
It is well known that Raman scattering is a valuable tool to identify chemical and biological samples through the detection of the characteristic oscillations of the molecules contained in these samples. However, because in normal conditions the probability of Raman scattering is very small, so the ability for the molecule identification of Raman scattering has only really become significant after the discovery of surface-enhanced Raman scattering (SERS). Within this phenomenon, molecules adsorbed onto a metal surface under certain conditions exhibit an anomalously large interaction cross-section for the Raman effect with the consequence that the Raman signal of these molecules is amplified by several orders of magnitude. Recently, SERS as a powerful analytical tool has been shown to have wide potential applications in biochemistry, chemical production, food safety and environmental monitoring [1–5].
As the name 'SERS' indicates, the amplification of the Raman signal strongly depends on the characteristics of the metal surface on which the analyte molecules are adsorbed. Metal surface that is used in order to amplify the Raman scattering signal of the analyte molecules when they are adsorbed onto it is commonly known as SERS-active substrate. Since the discovery of SERS, a key problem in the analysis application of SERS is to develop stable and reproducible SERS-active substrates that can provide as large as possible enhancement factor.
It was found that two kinds of enhancement mechanisms are involved for SERS. One is the chemical enhancement caused by the charge transfer between the metal surface and the adsorbed analyte molecules [6]. The other is the local electromagnetic (EM) enhancement that originates from the excitation of plasmons on the surface of metals [6–9]. Usually, the local EM enhancement is much higher than the chemical enhancement, and much more attention is paid to the structures that offer obvious local EM enhancements. Specifically, under appropriate circumstances, SERS enhancements as large as 1014 can be achieved [9], in which at least 8–10 orders of magnitude can arise from the EM effect, while the enhancement factor due to the chemical effect is only 101–102 times [10, 11].
The intensity of local EM field can be promoted by two kinds of nanostructures on the SERS substrates. One is a sharp tip with high curvature that acts as a 'lightning rod', and the other is a slit formed between neighboring metal nanoparticles that serve as a nanogap [12–14]. The regions of highly enhanced local EM field are often called 'hot spots'. The sharp tips and nanogaps usually are the main hot spots, and they play a decisive role in the enhancement of the Raman signal. The SERS-active substrates containing these two kinds of structures have been widely prepared. The metal dendritic nanostructures are a class of such substrates. For metal dendrites, the nanoscale junctions and the interstices of overlapped branches can become hot spots, while the branches and tips of dendritic structures have strong surface plasmon polariton, which promotes the enhancement of SERS. Therefore, the metal dendrites are expected to show high SERS activity.
Until recently, the most common method for the production of SERS substrates involved the synthesis of metal (silver or gold) colloids. Metal colloids, in general, show strong enhancement and are easily prepared and manipulated. A major problem with the use of metal colloids is the tendency to colloidal aggregation after the addition of analyte, which makes the colloids unstable and leads often to the poor reproducibility of the SERS spectra. At the same time, the aggregation of colloidal particles is a prerequisite for strong SERS enhancement [15, 16]. The dual nature of aggregation forces us to live with it. Due to the aforementioned disadvantages of metal colloids, in recent times there has been a strong shift to the use of SERS substrates that are made from metal nanoparticles or nanostructures immobilized on a solid matrix.
In this report we will present the preliminary results of the use of silver nanodendrites (AgNDs) deposited onto silicon (from now on this structure will be referred to as AgNDs@Si) as SERS substrates to detect trace concentrations of paraquat (PQ), a herbicide commonly used in Vietnam as well as in many other countries for weed control. PQ has been selected as a probe molecule for AgNDs@Si SERS substrates due to its toxicity to humans even at very low concentrations. In particular, PQ has toxic effects on human heart, liver, and brain tissue. PQ exposure has a high mortality rate attributed to a lack of effective treatments [17, 18]. Contributing to the severity of PQ toxicity is its high solubility in water [19]. Recently we had a report on the trace analysis of PQ using different SERS substrates [20]. In this paper we will present in more detail the use of AgNDs@Si to detect trace amounts of PQ. In the literature there was also a report on the trace analysis of PQ, using the SERS-based microdroplet sensor [21].
It should be noted that the solid matrix, which was used to secure the AgNDs in our study, is silicon. Silicon was chosen for two reasons. First of all, in most cases, silicon is neutral for the analyte molecules. Next, silicon is capable of allowing a relatively thick layer of AgNDs associated with its surface. In turn, this could happen because one can perform the silver deposition process simultaneously with silicon etching. The AgNDs have been deposited on Si either by electroless deposition, or by constant voltage electrodeposition, but both methods have been carried out in an aqueous solution of silver nitrate (AgNO3) and hydrofluoric acid (HF). In this solution AgNO3 is the source of silver ions to form AgNDs, while HF dissolves the initial native silicon oxide layer on the surface of silicon as well as dissolves silicon directly in the process followed. In the literature there were a number of research groups, which have been using electroless deposition with a similar solution to produce AgNDs on silicon [22–24]. However, we have only found two reports on the use of electrodeposition to fabricate AgNDs on silicon. Of these, one group of authors has used the aqueous colloidal silver solution (which was prepared by laser ablation) to produce AgNDs, accompanied by a constant current mode of electrodeposition [25]. The second research group performed the electrodeposition of AgNDs with constant voltage mode like us, but they used a solution containing additional NH3 component and they have observed that the NH3 concentration has strong influence on the formation of AgNDs [26].
2. Experimental
AgNDs have been manufactured either by electroless deposition, or by electodeposition at room temperature on the surface of silicon, using an aqueous solution of AgNO3 and HF. Silicon used was the boron-doped p-type single crystalline (100) Si with resistivity of 0.5–50 Ω.cm. The reagents used were of the analytical reagent grade with concentrations as follows: AgNO3 is 99.8%, HF is 40%. The water used was deionized water. The process of silver deposition on silicon using electroless method was carried out as follows. At first Si wafer was cleaned with acetone to remove grease. Next, the clean Si wafer was immersed in 5% HF solution for 30 min to achieve H-terminated silicon surface. The silicon wafer covered by Si–H bonds was then immediately placed into a freshly prepared reduction solution containing 4.8 M of HF and AgNO3 with different concentrations for 15 min. The different AgNO3 concentrations may produce different silver structures on silicon surface. Therefore, in order to find out the concentration of AgNO3 which will give dendritic structure, the following concentrations of AgNO3 were used: 0.25, 1.0, 2.5, 5.0, 10.0 and 20.0 mM.
The electrodeposition of AgNDs was carried out under the constant voltage mode using a typical electrodeposition set-up consisting of a potential source with a platinum grid anode and a silicon cathode. The two electrodes were placed parallel to each other and separated by a distance of 2 cm in a teflon container. Prior to electrodeposition, aluminum was evaporated on the back of the Si wafer to create an ohmic contact, this process allowed the Si wafer to be used as an electrode. The electrodeposition was performed with a constant voltage of 12 V and duration of 15 min. The solution used was an aqueous solution containing 20 mM of AgNO3 and 4.8 M of HF. The 20 mM concentration of AgNO3 was used in the silver electrodeposition, because during silver electroless deposition, this concentration already gave the silver dendritic structure.
The structure and morphology of representative AgNDs@Si samples were examined by scanning electron microscopy (SEM) using a S-4800 field emission scanning electron microscope (Hitachi, Japan). The SERS measurements of PQ were performed by dripping 50 μl of aqueous PQ solution with different concentrations onto the AgNDs@Si surface. The spreading area is ∼1 × 1 cm2. After PQ dripping, samples were let stand in air at room temperature until dry. Raman spectra were recorded only when the samples were dried. Raman spectra were recorded with a Jobin–Yvon LabRam Raman microscope with input laser light of 632.8 nm wavelength.
3. Results and discussion
The electroless deposition of silver onto silicon in an aqueous solution containing silver ions (Ag+) and HF is based on an electrochemical redox reaction in which both anodic and cathodic processes occur simultaneously at the silicon surface [27]. Electrochemical reactions are illustrated in the following equations:
anodic:
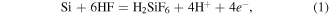
cathodic:
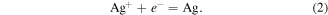
In the reactions above e− denotes electron (with a negative charge). H2SiF6 is a soluble compound, so the reaction (1) is the reaction of the Si removing by etching. Meanwhile the reaction (2) is the reaction of the reduction of ionic silver to atomic silver on the silicon surface. Our studies have shown that silver dendrites will only be formed when the concentration of AgNO3 in the deposition solution is large enough. This is illustrated in figure 1. Figure 1 shows the SEM images of the silver particles or structures that have been formed on a silicon surface after a silver electroless deposition process for 15 min at room temperature, using a solution containing 4.8 M of HF and different AgNO3 concentrations, including 0.25, 1.0, 2.5, 5.0, 10.0 and 20.0 mM. In figure 1 we can see that when the concentration of AgNO3 was still low (0.25 mM—figure 1(a)), nano-sized silver particles with nearly round shape grow on the surface of silicon. When the concentration of AgNO3 increased to 1.0 mM (figure 1(b)), the silver particles are spread out horizontally, forming a silver layer that covered almost the entire surface of the silicon. Next, when AgNO3 concentration was 2.5 mM (figure 1(c)), a new silver nanoparticle layer is grown on the top of lying-on-surface silver layer. A special feature of the new layer is that this layer has some silver nanoparticles elongated in a certain direction. When the concentration of AgNO3 is 5 mM (figure 1(d)), the above process of on-top growing and elongation continues to occur, along with the size of the silver particles has become larger. At 10 mM of AgNO3 (figure 1(e)), we see clearly that the silver nanorods (with a length of a few tens μm) was formed on the silicon surface. Finally, at 20 mM of AgNO3 (figure 1(f)), the side branches grow out from the silver nanorods, which means that the silver nanodendrites were formed.
Figure 1. SEM images of silver particles or structures which have been electrolessly deposited on silicon for the same 15 min in solutions with the same 4.8 M HF concentration, but with different AgNO3 concentrations: (a) 0.25 mM, (b) 1.0 mM, (c) 2.5 mM, (d) 5.0 mM, (e) 10.0 mM and (f) 20.0 mM.
Download figure:
Standard image High-resolution imageIn our opinion, the sprouting and growth of silver dendritic structure as above can be explained by the diffusion-limited aggregation model [28]. According to this model, at first silver nanoparticles generated in the deposition process will hit and stick with each other, thus forming initial aggregates of silver nanoparticles. Then, more and more free nanoparticles will diffuse toward the aggregates to form larger aggregates by the continuous hitting and sticking processes. At a certain stage, the backbones of the dendrites are formed. As the reaction proceeds, the growth is mainly driven by the decreasing surface energy, and the growth of the nanostructure prefers to occur at tips and stems of the branches. Thus the dendritic Ag nanostructures are formed by anisotropic growth of the aggregates.
Figure 2 shows the SEM images with two magnifications of two AgNDs sets that have been deposited on Si, both within 15 min, in the same aqueous solution containing 20 mM of AgNO3 and 4.8 M of HF, but using two different methods: electroless deposition (figures 2(a) and (b)) and electrodeposition with 12 V constant voltage (figures 2(c) and (d)). The results showed that the electrodeposited AgNDs have much better ramification. Specifically, on a trunk, the number of branches becomes much greater, the branches are almost of the same length, forming the same angle with the trunk and evenly spaced. Besides, the density of the dendrites also becomes thicker and dendrites arranged together in a better order.
Figure 2. SEM images with two magnifications of two AgNDs sets that have been deposited on Si, both within 15 min, in the same aqueous solution containing 20 mM of AgNO3 and 4.8 M of HF, but using two different processes: electroless deposition ((a) and (b)) and 12 V constant voltage electrodeposition ((c) and (d)).
Download figure:
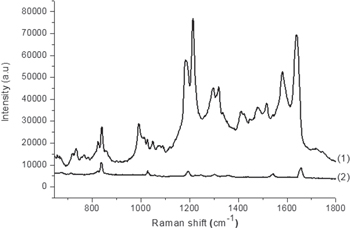
Standard image High-resolution imageOn the next step, the AgNDs sets, which have been manufactured as described above, were used as SERS substrates to detect trace amounts of PQ. The result of this work is illustrated by figure 3. Figure 3 shows the Raman spectra of PQ with a concentration of 5 ppm dripped onto two AgNDs@Si samples, which have been manufactured during the same 15 min, in the same aqueous solution of 20 mM of AgNO3 and 4.8 M of HF, but with two different processes: electroless deposition (curve (1)) and 12 V constant voltage electrodeposition (curve (2)). In figure 3 we can see clearly that the Raman spectrum of PQ, which was recorded by AgNDs electrodeposited on silicon, is of much higher amplification than the one that was recorded by electroless deposited AgNDs. More specifically, in the Raman spectrum of PQ recorded by electrodeposited AgNDs@Si, the number of peaks obtained is more, the intensity of the peaks is higher, and the separation between the peaks is better, compared to the peaks of PQ recorded by electroless deposited AgNDs@Si. It should be noted that in accordance with the data of [29], all well-separated and strong peaks on the curve (1) of figure 3 are the Raman peaks of PQ molecule. Thus, on the curve (2), due to poor separation and low intensity, some of the characteristic peaks of the PQ molecule have disappeared in the Raman spectrum recorded by electroless deposited AgNDs@Si.
Figure 3. Raman spectra recorded in the same conditions of PQ with 5 ppm concentration dripped onto two AgNDs@Si samples, which have been manufactured for the same time period (15 min) in the same solution (20 mM of AgNO3 and 4.8 M of HF) by (1) electroless deposition and (2) electrodeposition.
Download figure:
Standard image High-resolution imageThe combination of figure 2 with figure 3 shows clearly the correspondence between the morphology of dendrites and the amplification of a Raman spectrum, as we were expecting. This fact can be explained on the basis of the increase in the number of 'hot spots'. In the SERS substrates fabricated by electrodeposition of AgNDs on silicon, due to the better ramification of dendites, the number of sharp 'lightning' tips as well as of 'nanogaps' become more. Therefore the number of 'hot spot' to amplify the Raman signal has become much greater. As a result, the SERS enhancement of electrodeposited AgNDs@Si substrate has have become significantly higher compared with that of the electroless deposited AgNDs@Si substrate.
To find out the detection limit for PQ of AgNDs@Si fabricated by electrodeposition, we have used the AgNDs@Si samples which were prepared in identical conditions (12 V constant voltage electrodeposition for 15 min in the aqueous solution containing 20 mM of AgNO3 and 4.8 M of HF) to record the Raman spectra of PQ with different concentrations, including 1, 0.5, 0.1 and 0.01 ppm. The result is shown in figure 4. From figure 4 we can see that the minimum concentration of PQ, which the electrodeposited AgNDs@Si SERS-active substrate can detect, is ∼0.01 ppm. Meanwhile, this limit is ∼5 ppm for the electroless deposited AgNDs@Si substrate (figure 3). This means that by changing the AgNDs@Si fabrication method, the detection limit for PQ was increased by about two orders of magnitude. This also demonstrates that the surface morphology of the AgNDs plays a crucial role in determining the magnitude of the SERS enhancement. Moreover, from figure 4 we can see that the reproducibility of fabricated AgNDs sets is very good. This is manifested by the fact that when we dripped PQ on AgNDs with decreasing concentrations, we also have obtained Raman spectra of PQ with the peaks with decreasing intensity and resolution, respectively, as shown in figure 4.
Figure 4. Raman spectra of PQ with different concentrations: (1) 1 ppm, (2) 0.5 ppm, (3) 0.1 ppm and (4) 0.01 ppm dripped on identical electrodeposited AgNDs@Si samples.
Download figure:
Standard image High-resolution image4. Conclusions
In conclusion, the AgNDs deposited on silicon have been manufactured by two methods: electroless deposition and electrodeposition, using the same aqueous solution of AgNO3 and HF, and after that they have been used as SERS-active substrates to detect trace amounts of PQ, a commonly used herbicide. The results showed that the electrodeposited AgNDs have much better ramification. Specifically, on a trunk, the number of branches becomes much greater, the branches are almost of the same length, forming the same angle with the trunk and evenly spaced. Besides, the density of the dendrites also becomes thicker and dendrites arranged together in a better order. Corresponding to the improvement of AgNDs morphology is the improvement of the intensity and resolution of the SERS spectrum of PQ, when the AgNDs with improved morphology were used as SERS substrates. As a result, while the SERS-active substrates made from electrodeposited AgNDs were able to detect PQ with concentration as low as 0.01 ppm, the ones made from electroless deposited AgNDs could only detect the concentration of PQ hundreds of times higher.
Acknowledgments
This work was supported financially by the National Key Laboratory for Electronic Materials and Devices (located at the Institute of Materials Science). The authors express sincere thanks to Professor Nguyen Van Hieu for the valuable support.