Abstract
Secreted carbohydrates by Chlorella vulgaris cells were used for reducing and capping Silver nanoparticles (AgNPs). Oxygen-bearing functional groups on the carbohydrates found to be the main biochemical groups responsible for anchoring the metal nanoparticles. Transmission electron microscopy (TEM) micrographs showed that isotropic small particles with mean particles size of 7 nm were synthesized. Comparing the TEM results with DLS analysis revealed that the thickness of carbohydrate capping was about 2 nm. A zeta potential of +26 mV made the particles colloidally stable and desirable for anticancer and antimicrobial applications. The MIC against gram positive (Staphylococcus aureus) and gram negative bacteria (Escherichia coli) were determined to be 37.5 μg ml−1 and 9.4 μg ml−1, respectively. Treatment of Hep-G2 cells with 4.7 μg ml−1 AgNPs for 24 h reduced the cell viability to 61%. This concentration was also reduced the cell viability to 37% after 48 h of exposure.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Silver nanoparticles (AgNPs) are among the most common and applicable nanostructures. These materials have gained various applications such as antimicrobial wound healing and burn treatment agents, molecular labeling for high sensitivity bio-molecular detection and diagnostics, dental resin composites, coating of medical devices, nonlinear optics, optical receptors and electrical batteries [1–6].
There are several chemical methods for synthesis of silver nanoparticles, but these methods usually employ extreme and harsh conditions. Chemical methods result in accumulation of toxic chemicals on the surface of nanoparticles. This phenomena significantly limits the medical applications of AgNPs [7]. Furthermore, chemically synthesized nanocrystalline silver colloids are not stable and are vulnerable to aggregation [5]. Therefore, capping (stabilizing) agents should be used to increase the stability of the prepared nanoparticles. Particularly in the synthesis of AgNPs via reduction of Ag+ ions factors such as choice of solvent, type of reducing agent and the capping (stabilizing) agent play a key role.
Bioactive compounds from microorganisms and plants have a valuable capability for reduction and capping of AgNPs without using any toxic chemicals and harsh reaction conditions [8–11]. Carbohydrates and proteins from microbial cells can be effective in reduction of Ag+ ions [12–14]. Likewise, biochemical compounds in plant extracts such as ketones, aldehydes, polyphenols, caffeine and isoverbascoside can function as reducing and capping agents for AgNPs biosynthesis [8–11]. Use of biological compounds for synthesis and production of nanoparticles have gained significant interest due to several advantages namely low cost and mild synthesis conditions. Biological methods do not produce any toxic material, have no adverse effect on the environment and are economic for scale up [15, 16].
Microorganisms have a capability to reduce metal ions via resistance and detoxification mechanisms [17]. Many microorganisms such as Morganella, Pseudomonas, Bacillus, Lactobacillus, Geobacillus, Escherichia, Vibrio, Salmonella, Klebsiella, Streptomyces, Rhodococcus, Cladosporium, Fusarium, Aspergillus, Candida and Penicillium have been used for the green synthesis of AgNPs [7, 15, 16, 18–25]. To date, main organisms that have been used for biosynthesis of nanoparticles are consisted as bacteria and fungi, which may be pathogenic to human. So, there is an increasing interest in the development of the AgNPs biosynthesis by using algae [26–28]. Algae do not need complicated carbon and nitrogen sources. They use sunlight as energy source, carbon dioxide as carbon source and ammonium salts as nitrogen source. So these organisms are completely economic for biotechnological processes in large scales [29].
Among microalgae Chlorella vulgaris is one of the most applicable and beneficial species. It is a popular food source because of its protein, lipid, vitamin and other essential nutrient content [30–32]. Currently, there is a large scale culturing of this microalgae for production of Chlorella vulgaris biomass. Consequently, vast amounts of the culture supernatant are produced as waste product which results in environmental pollution. Previously cell extracts of Chlorella vulgaris have been used as a source of bioactive compounds for reducing silver ions to AgNPs. Proteins in the cell extract were involved in the biosynthesis by providing dual function in reduction and shape-controlling of the synthesized AgNPs [14]. Here for the first time we applied culture supernatant of Chlorella vulgaris as the waste by-product from biotechnological industries, for biosynthesis of colloidally stable AgNPs.
2. Materials and methods
2.1. Algae cultivation and supernatant preparation
Chlorella vulgaris cells were inoculated in 50 ml BG-11 broth medium (107 cells ml−1) in a 250 ml erlenmeyer flask and incubated at 27 °C under continues illumination with fluorescent lamps at 60 μEm−1 s−1 intensity [29]. The cells growth curve was drawn using a Neubauer chamber.
2.2. Biosynthesis of AgNPs
After 20 days incubation, corresponding to the end of logarithmic phase of growth, the culture was centrifuged and the supernatant was used for biosynthesis reaction. In practice, silver nitrate solution and culture supernatant were preheated up to 50 °C. Then, 15 ml culture supernatant was added to 30 ml silver nitrate solution, the final AgNO3 concentration was adjusted to be 5 mM. The reaction mixture was incubated at 50 °C in a water bath for 24 h with no stirring or shaking.
2.3. Characterization of AgNPs
The AgNPs suspension was diluted ten times with deionized water and evaluated by UV–vis spectrometer (T80+ UV/VIS Spectrometer PG Instruments Ltd) operated at a resolution of 1 nm within the range of 300–700 nm [33]. A drop of prepared AgNPs suspension was dripped on a copper grid and dried at room temperature. Transmission electron microscopy (TEM) micrographs obtained with Philips EM 208S, TEM, operated at HT 100 kV. For Fourier transform infrared (FTIR) spectroscopy analysis, prepared nanoparticles were centrifuged and washed with deionized water and dried in an oven at 50 °C overnight. FTIR spectroscopy analysis was done on a Bruker, Vertex 70, FTIR spectrometer using KBr pellets [34–38]. The crystallinity of the particles was evaluated by x-ray diffraction patterns (XRD, Siemens D5000) using drop coated films on a glass slide [11, 39, 40]. Particle size distribution and zeta potential analysis were performed by dynamic light scattering (DLS) studies on a Malvern, ZS3600 instrument.
2.4. Determination of biochemical compounds in culture supernatant
Microbial cells secrete carbohydrates and proteins to the extracellular environment which can be effective in reduction of Ag+ ions [12–14, 29, 41–44]. So, the carbohydrate and protein content of culture supernatant were determined to evaluate if there is any biological compounds with potential for bioreduction of AgNPs. The phenol-sulfuric acid assay was used for carbohydrate content determination [45–47]. In this order, 100 μl of 5% phenol in water was mixed with 100 μl of culture supernatant. Subsequently, 400 μl of concentrated sulfuric acid was injected to the mixture and the tube mixed quickly. The reaction was allowed to reach the maximum temperature and stand for 10 min. Then, incubated in water bath at room temperature for 30 min. Light absorbance was read at 490 nm on a spectrophotometer (T80+ UV/VIS Spectrometer PG Instruments Ltd). The carbohydrate content was determined as equal glucose concentration by reference to a standard curve for D-glucose.
The amount of protein present in the culture supernatant was determined by performing a colorimetric reaction and comparing the results with those obtained from standard amounts of albumin. The Bradford micro-assay test was used which is generally used for dilute protein solutions. In practice, 20 μl of the concentrated dye reagent was added to 80 μl of the culture supernatant. The mixture was shaken and incubated at room temperature for 5 min. Finally, light absorbance at 595 nm is read using UV–vis spectrometer. The dye was prepared by dissolving 5 mg coomassie brilliant blue G-250 in 0.5 ml ethanol 95%. Then, 1 ml 85% (w/v) phosphoric acid was added and the solution was dilute to 10 ml.
2.5. Cytotoxicity assay
Cytotoxicity of synthesized silver nanoparticles was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using Hep-G2 cell line in a 96-well plate. In experiment, Hep-G2 cells (1 × 106 cells) were suspended in 15 ml RPMI 1640 media containing 10% FBS. Cell suspension was then aliquoted in 96-well plate, 150 μl (1.5 × 104 cells) in each well. The plate was incubated at 37 °C and 5% CO2. After 24 h the media were replaced by fresh media containing desired concentration of AgNPs in the presence of blank and control. After 24 and 48 h incubation, the media were replaced by 25 μl MTT solution (4 mg ml–1 media) and cells were incubated for another 3 h. To dissolve the formazan crystals 100 μl DMSO was added to each well and plate was shaken for 1 min and absorbance was read at 540 nm by microplate reader (BioTek, Power Wave XS2) [38].
2.6. Antimicrobial test
One species each of a gram positive (Staphylococcus aureus) and gram negative bacteria (Escherichia coli) were used for the antibacterial activity assay. Minimum inhibitory concentration (MIC) was determined using standard microdilution method according to the protocol of the Clinical and Laboratory Standards Institute (CLSI M07-A8) [2, 48]. The bacteria cells were cultured in Mueller-Hinton broth up to turbidity of the barium sulphate 0.5 McFarland standards at an OD600 of 0.12. Then the cell suspensions were diluted 1:20, 10 μl of diluted bacterial suspension was inoculated to 90 μl Mueller-Hinton broth with the desired concentration of AgNPs in each well of a 96-well plate. For control wells, Mueller-Hinton broth without any nanoparticles was used and wells with no bacteria inoculation served as blanks. After 24 h incubation at 37 °C, the OD was read by microplate reader (BioTek, Power Wave XS2) at 600 nm.
3. Results
3.1. Biosynthesis of AgNPs
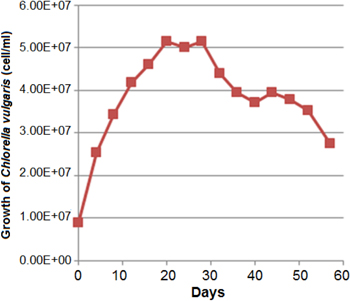
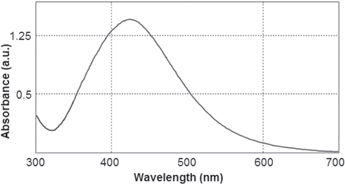
The growth curve of Chlorella vulgaris cells through 60 days is shown in figure 1. After 20 days of incubation, cells were at the end of their logarithmic growth and on day 28 the cells were at the end of stationary phase. Interestingly, after 40 days of incubation a second growth was observed as shown in figure 1. Our investigations about the effect of Chlorella vulgaris growth phase on the biosynthesis of AgNPs indicated that the best result was obtained at the end of logarithmic phase (day 20). At this stage addition of the culture supernatant to aqueous silver nitrate solution was resulted in formation of yellowish brown color in few minutes. Accordingly, the solution color was changed to dark brown after 24 h (figure 2). UV–vis analysis of the prepared silver nanoparticles exhibited single surface plasmon resonance (SPR) peak at 434 nm without any shoulder (figure 3), which is the characteristic optical feature of monodisperse isotropic AgNPs [14]. Poly-dispersed AgNPs and non-uniform nanoparticles exhibited multiple SPR bands or bands with shoulders [14]. It is worth to mention that, stirring or shaking prevented the formation of AgNPs and subsequently no color change was observed in the reaction mixture.
Figure 1. Growth curve of Chlorella vulgaris.
Download figure:
Standard image High-resolution imageFigure 2. Reaction color before (a) and after (b) bioreduction of AgNPs.
Download figure:
Standard image High-resolution imageFigure 3. UV–vis light absorption spectrum of the prepared AgNPs.
Download figure:
Standard image High-resolution image3.2. Determination of biochemical compounds in culture supernatant and characterization of AgNPs
The total carbohydrate in culture supernatant at the end of logarithmic growth phase of Chlorella vulgaris was equal to 0.15 mg ml−1 glucose. Also, protein content was determined to be equal to 7.16 μg ml−1 albumin. After biosynthesis of AgNPs, three-fold reduction in the carbohydrate content was observed. The carbohydrate content of the supernatant after reduction reaction was equal to 0.05 mg ml−1 glucose. While, there was not any change in the protein concentration. In comparison with previous study on Chlorella vulgaris cell extract in which proteins were found to be the main molecules for AgNPs bioreduction [14], current data are showing that carbohydrates are the effective secretory compound for reducing and stabilizing of AgNPs.
It is widely believed that oxygen-contain functionalities are necessary for anchoring of metal nanoparticles to biochemical compounds, and silver ions can easily oxidize these groups as shown in equations (1) and (2) [35, 49–51]. Commonly, reduction of Ag+ ions occurs via oxidation of hydroxyl groups to carboxyl [49, 50]. Carbohydrates are abundant with oxygen-bearing functional groups, such as hydroxyl, carboxylic, carbonyl and phenolic groups. Silver ions have a strong affinity to these functional groups via electrostatic or coordination interactions. These interactions allow electrons to pass to the Ag+ ions resulting in nucleation and growth of AgNPs:
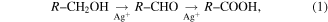
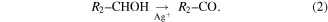
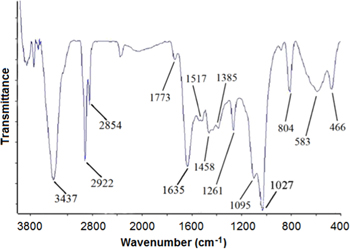
Representative FTIR spectrum of the obtained AgNPs showed the key role of oxygen containing functional groups (figure 4). The 3437 cm−1 band is assigned to the O−H groups [34–36, 52, 53]. The bands at 2922 and 2854 cm−1 can be due to the presence of aliphatic C−H groups [37, 38]. The absorption peak corresponding to carbonyl groups stretching vibration was appeared at 1635 cm−1. The carbon–carbon double and single bonds were appeared at 1517 cm−1 and 1261 cm−1, respectively [54]. The characteristic of strong peak of C−O band can be seen at 1027 cm−1.
Figure 4. FTIR spectrum of the biosynthesized AgNPs.
Download figure:
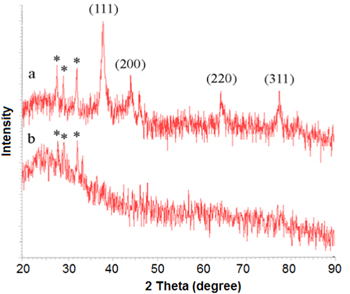
Standard image High-resolution imageThe powder diffraction pattern of the AgNPs is depicted in figure 5. Four main characteristic diffraction peaks for silver were observed at 38.2°, 44.5°, 64.4° and 77.5° of 2θ values due to reflection from the crystal facet of (111), (200), (220) and (311), respectively [33, 55, 56]. Also, there are peaks at 28.0°, 29.4° and 32.4° of 2θ values that are indicated by asterisks. Some researchers have justified that these peaks are due to the interaction of silver nitrate with biologic matrixes [57, 58]. Meanwhile, similar peaks also can be seen in XRD spectrum recorded from 5 mM AgNO3 in BG-11 medium (curve (b) in figure 5). Therefore, the presence of these peaks can be attributed to mineral complexes such as Ag3PO4 [59].
Figure 5. XRD patterns of (a) AgNPs and (b) 5 mM AgNO3 in BG11 medium, four main characteristic peaks of silver indexed to the (111), (200), (220) and (311). Asterisks (*) indicate the peaks attributed to mineral complexes, such as Ag3PO4.
Download figure:
Standard image High-resolution imageStudies on particles shape and size were performed by TEM analysis (figure 6). Mean particle size was determined to be 7 nm. Prepared particles were fairly uniform and spherical in shape, indicating the isotropic growth of silver nanocrystals. Some hallows were seen around nanoparticles which can be due to the biochemical materials responsible for reduction and capping of AgNPs.
Figure 6. TEM micrograph of the biosynthesized AgNPs.
Download figure:
Standard image High-resolution imageDLS analysis has provided further insight into size detail and zeta potential of the prepared particles. Figure 7 shows the particle size distribution and zeta potential diagrams. Further data on DLS analysis are presented in table 1. Mean particle size was 9 nm, indicating that the prepared AgNPs are uni-dispersed in aqueous matrices. The 2 nm increase in measured hydrodynamic diameter in relation to TEM micrographs is possibly due to carbohydrate coating. The prepared AgNPs posed a positive zeta potential of +26 mV which implies a stable colloidal system. Nanoparticles with surface charges greater than +25 mV or less than 25 mV are considered as colloidal stable [9]. Positive surface charge enhances electrostatic attraction between the AgNPs and negatively charged cell membranes [60]. This synergistic effect will significantly increase the antimicrobial properties of the AgNPs [61]. Synthesized AgNPs were stable over six months at room temperature with no observable flocculation or sedimentation traces.
Figure 7. Particles size distribution (a) and zeta potential (b) diagrams of AgNPs.
Download figure:
Standard image High-resolution imageTable 1. DLS data for prepared AgNPs in DI water.
| Size distribution (nm) | Mean size (nm) | Zeta potential (mV) | pH | Temperature (°C) |
|---|---|---|---|---|
| 6.5–13.5 | 9 | +26 | 7.4 | 25 |
3.3. Cytotoxicity assay and antimicrobial tests
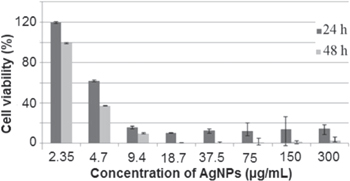
Cytotoxic effects of the biosynthesized AgNPs were examined over 24 and 48 h exposure to Hep-G2 cell line by MTT assay. Hep-G2 cells treatment with 4.7 μg ml−1 AgNPs for 24 h was reduced the cell viability to 61%. This concentration was also reduced the cell viability to 37% after 48 h of exposure (figure 8). Despite the type of cell line, anticancer effect of AgNPs is also dependent to the characteristics and concentration of nanoparticles, cell environment and exposure time [62–65]. Particularly for Hep-G2 cell line, according to the nanoparticles properties, the MIC of AgNPs after 24 h of exposure could be changed from 3 to 55 μg ml−1 [62, 63, 66].
Figure 8. Cytotoxicity assay of the prepared AgNPs against Hep-G2 cell line.
Download figure:
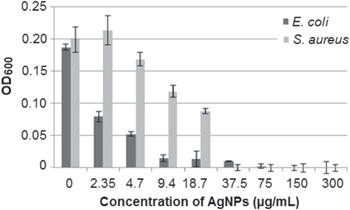
Standard image High-resolution imageSilver ions and silver based compounds have strong antimicrobial effects and have been used for decades as antimicrobial agents in various fields [67, 68]. AgNPs provide high fraction of exposed atoms due to their extreme small size and thus expand the contact surface of silver with microorganisms. In our experiment, prepaired nanoparticles have shown intense effects on the bacterial cells. The MIC concentrations against S. aureus and E. coli were determined to be 37.5 μg ml−1 and 9.4 μg ml−1, respectively (figure 9). These concentrations are acceptable in contrast to previously reported MIC values (10–60 μg ml−1) of AgNPs against S. aureus and E. coli [1, 69, 70].
Figure 9. Antimicrobial effects of AgNPs against E. coli and S. aureus.
Download figure:
Standard image High-resolution imageThe toxic effects of AgNPs is broadly similar to that of Ag+ ion and accurately follows the dose response pattern of Ag+ ion [68, 71]. However, there is a debate on the mechanisms of action by which AgNPs exert toxicity to prokaryotic and eukaryotic organisms. The most elusive question is whether AgNPs exert direct particle-specific effects beyond the known antimicrobial activity of released Ag+ ions. Xiu et al have ruled out direct particle-specific biological effects by showing the lack of antimicrobial properties of AgNPs under anaerobic conditions [68]. Silver ions can penetrate into the bacterial cell, which can oxidize the cellular biomolecules and also facilitating the generation of reactive oxygen species and consequently damaging the cell. The importance of released Ag+ ions in the AgNPs cytotoxicity was also reported. AgNPs which release more Ag+ ions are more toxic for hepatocytes [72]. On the other hand, nanoparticles can bind to the cells and make cell membranes more permeable. The added nanoparticles diffuse to the surface of the membrane and are presumably adsorbed and diffused within the membrane. Step by step the membrane conductance is increased. This process is a dynamic, reversible and the pore-forming substance can leave the membrane which results in conductance decrease [73]. Energy-filtering TEM studies showed the considerable changes in the cell membranes upon treatment with AgNPs [71]. Disruption of membrane morphology may cause a significant increase in permeability, leading to uncontrolled transport through the plasma membrane and finally cell death.
4. Discussion
In the present study, isotropic, small and uniform AgNPs were synthesized through a biosynthesis reaction using Chlorella vulgaris secretory carbohydrates. Biosynthesis of AgNPs by using intracellular peptides from Chlorella vulgaris cells have been reported previously. The authors mentioned anisotropic growth of AgNPs as the main production challenge. Oval, truncated triangular and triangular nanocrystals were formed behind the spherical particles [14].
AgNPs are known to exhibit size and shape dependent SPR. Anisotropic nanocrystals exhibit multiple SPR bands that influence the optical properties of the product [14, 39]. Biochemical compounds can provide dual function namely (i) for Ag+ ion reduction and (ii) controlling the AgNPs shape. Some chemical functional groups such as hydroxyl and phenolic groups act as reducing agents while carboxyl groups can posse shape-directing functionality and can promote the anisotropic growth of AgNPs. Spherical particles could be produced through amination modification, which converts all of the carboxyl groups into amine groups. Amination modification is a labor and time intensive task and is not a perfect solution as still some anisotropic nanocrystals will form. Correspondingly, the synthesis of small nanoparticles is a challenge and usually large particles are produced during a bioreduction reaction [14].
The chemistry of biological reducing compound is not the single role playing factor in the biosynthesis reaction. Factors such as concentration of silver precursor, reaction temperature and concentration of reducing and capping agent are also among the main influential factors which affect the pattern of crystal growth and quality of the prepared particles [74–76].
More recently, there is much more interest for secretory bioactive compounds as compared to intracellular compounds for biotechnological processes. Using secretory molecules significantly shortens the process time and reduces the associated costs. In the case of Chlorella vulgaris cells, the biomass is currently used as the health supplement and for biodiesel production [30, 31]. Therefore, incorporation of the produced waste from these industries provides a cheap and sustainable source for the biotechnological production of AgNPs.
5. Conclusions
Chlorella vulgaris cells secret carbohydrates in logarithmic growth phase which are effective in AgNPs biosynthesis. Using secretory carbohydrates has many advantages over the previously used intracellular proteins. These include the ease of production and synthesis of small isotropic nanoparticles. Oxygen containing functionalities on the carbohydrates are the active sites for anchoring the metal nanoparticles and silver ions can easily oxidize these biochemical groups. The carbohydrate capping provides a positive zeta potential for the AgNPs which is a critical characteristic for interactions with biologic membranes. In addition, the prepared particles in this study were so uniform having the mean particle size of 7 nm. These unique characteristics provide large surface area for Ag+ ions release and their future industrial application.
Acknowledgments
This work was financially supported by the School of Pharmacy and Pharmaceutical Sciences Research Centre, Shiraz University of Medical Sciences, Shiraz, Iran.