Abstract
HER-2/ErbB2/Neu(HER-2), a member of the epidermal growth factor receptor family, is specifically overexpressed on the surface of breast cancer cells and serves a therapeutic target for breast cancer. In this study, we aimed to isolate DNA aptamer (Ap) that specifically bind to a HER-2 overexpressing SK-BR-3 human breast cancer cell line, using SELEX strategy. We developed a novel multifunctional composite micelle with surface modification of Ap for targeted delivery of paclitaxel. This binary mixed system consisting of Ap modified pluronic®F127 and chitosan could enhance PTX loading capacity and increase micelle stability. Polymeric micelles had a spherical shape and were self-assemblies of block copolymers of approximately 86.22 ± 1.45 nm diameter. PTX could be loaded with high encapsulation efficiency (83.28 ± 0.13%) and loading capacity (9.12 ± 0.34%). The release profile were 29%–35% in the first 12 h and 85%–93% after 12 d at pH 7.5 of receiving media. The IC50 doses by MTT assay showed the greater activity of nanoparticles loaded paclitaxel over free paclitaxel and killed cells up to 95% after 6 h. These results demonstrated unique assembly with the capacity to function as an efficient detection and delivery vehicle in the biological living system.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Paclitaxel (PTX), the first of a new class of microtubule stabilizing agents, has demonstrated significant antitumor activity in clinical trials against a broad range of solid tumors, including refractory ovarian cancer, metastatic breast cancer, non-small-cell lung cancer, AIDS-related Kaposi's sarcoma [1, 2]. PTX is a highly hydrophobic diterpenoid pseudo alkaloid with poor aqueous solubility of approximately 1 μg ml−1. To solve this problem, PTX has to be formulated (Taxol) at 6 mg ml−1 in a vehicle composed of 1:1 blend of Cremophor EL and ethanol, which can be administered by intravenous injection [3]. However, this administration method can cause great distress to patients and is inconvenient for them. Because of the relatively large amount of Cremophor EL used and the nonspecific biodistribution of the drug in tumors and normal tissues, Taxol has also been associated with serious side effects, including severe hyper-sensitivity reactions, myelosuppression, and neurotoxicity. Developing a convenient and safe delivery system for PTX to maximize the therapeutic efficacy at tumor sites while minimizing the side effects is therefore a challenging endeavor.
In recent years, there have been considerable interests in developing biodegradable nanoparticles (NPs) as effective drug delivery systems [4]. Amphiphilic block copolymers have been widely investigated as hydrophobic drug solubilizing agents in drug delivery systems. They can spontaneously self-assemble into polymeric micelles and NPs in aqueous environments [5]. Most polymeric micelles are composed of a hydrophobic block as the inner core and a hydrophilic block as the outer shell. A hydrophobic drug can be encapsulated in the hydrophobic core of the micelles to increase the water solubility. The hydrophilic shell is able to prolong the circulation time due to a decrease in phagocytosis and renal clearance [6]. The polymeric micelles normally have average size of approximately 10–100 nm diameters, allowing the particles to accumulate in tumor tissue through a mechanism called enhanced permeation and retention effect rather than in normal tissues [7]. This is due to the fact that tumor vessels are structurally irregular and leaky compared to normal vessels. One of the most commonly used micelles in drug delivery applications is pluronic, an amphiphilic tri-block copolymer, composed of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO). The hydrophilic PEO and hydrophobic PPO blocks form the corona and the core of the micelles, respectively. Pluronic®F127 (PF) has attracted a lot of attention because of their low toxicity in the body and the ability to encapsulate any hydrophobic agents. However, the major problem of using polymeric micelles is their instability [8]. To overcome this limitation, grafting pluronic with chitosan (Chi) to form a copolymer was suggested. Chi is the cationic polysaccharide derived from chitin which stimulates cell growth and protein adsorption. Chi has been widely used in biomedical and pharmaceutical applications because of its biocompatibility and biodegradability [9]. Although Chi graft pluronic has been used in many forms such as hydrogel, nano-aggregation, and NPs, it has never been used as a delivery vector for anti-cancer drugs.
Aptamers are short single-stranded oligonucleotides of about 100 nt that bind their targets with high affinity and specificity [10]. They are randomly synthesized based on nucleic acid libraries by a procedure termed systematic evolution of ligands by exponential enrichment (SELEX) [11]. The attractive features of aptamers including a relatively small physical size, lack of immunogenicity in vivo, easy and reproducible synthesis, high-binding affinity and molecular specificity to their ligand, easy modification, fast tissue penetration, low toxicity, and long-term stability make them very good candidates for clinical applications and ideal alternatives for antibodies in numerous applications [14]. Owing to these unique characteristics, aptamers have been widely used to modify drugs and NPs for cancer therapy.
In this work total DNA aptamers were selected for a model SK-BR-3 breast cancer cell-line. The cell surface targets of the aptamers were also briefly investigated. Using an ionic-gelation method we synthesized and characterized novel NPs containing DNA aptamers for PTX delivery systems comprising Chi and PF. The properties of these polymeric micelles such as their appearance and stability, encapsulation efficiency, loading capacity (LC), and in vitro drug release are investigated. Especially, for the comparison of the cytotoxicity level on SK-BR-3 breast cancer cell-line, NS-VN-67 colon cancer cells and HT-VN-26 stomach cancer cells of Vietnamese patient were also evaluated.
2. Materials and methods
2.1. Materials
Chitosan F-MMW 400 kDa was purchased from Sigma Aldrich (USA). Pluronic®F127 (PF), paclitaxel (PTX), 3-(4,5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT), McCoy's 5A medium, Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS) and trypsin-ethylenediaminetetra-acetic acid (EDTA) were purchased from Life Technologies (Singapore). Slide-A-Lyzer™ dialysis cassettes with molecular weight cut-off (MWCO) of 3.5 kD was purchased from Thermo Fisher Scientific (USA). Oligonucleotide library 5'-TCA CCG GGA GGA GAC CCT GA-N40-GTG GCT TGG TGG TGG TTC AA -3'. Forward primer 5'-TCA CCG GGA GGA GAC CCT GA-3' and reverse primer 3'-TTG AAC CAC CAC CAA GCC AC-5' were synthesized by IDT (Singapore). SK-BR-3 breast cancer cell-line (HTB-30TM) was supplied from ATCC® (USA). Ceftriaxon was purchased from Bidiphar JSC (Vietnam). Sodium nitrite (NaNO2), hydrochloric acid (HCl), sodium hydroxide (NaOH), acetone, acetic acid, sodium triphotphate (TPP), succinic anhydride (SA), 4-dimethylaminopyridine (DMAP), triethylamine (TEA), N,N-dimethylformamide (DMF), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), N-hydroxysuccinimide (NHS), 2-mercaptoethanol, diethyl ether, dimethylsulfoxide (DMSO) were all analytical reagent purchased from Merck Milipore Chemical Company (Germany). All other chemicals were of analytical grade and used without further purifiation.
The colon cancer tissues and stomach cancer tissues of Vietnamese patients were kindly provided by the Oncology Hospital in Ho Chi Minh City. NS-VN-67 and HT-VN-26 cell-lines in DMEM containing 10% (v/v) FBS and 0.25 μg ml−1 ceftriaxon at 37 °C, 5% CO2 and 95% humidity, respectively, were cultured and collected.
2.2. SELEX procedures
SK-BR-3 cell-line was harvested by trypsinization and 1 × 106 cells were recovered in complete media at 37 °C for 30 min. 200 pmol ssDNA pool dissolved in 500 μl of binding buffer (4.5 g l−1 glucose, 5 mM MgCl2, 0.1 mg ml−1 yeast tRNA, 1 mg ml−1 bovine serum albumin in phosphate buffered saline (PBS)) was denatured by heating at 95 °C for 5 min and cooled on ice for 10 min before binding. Then the ssDNA pool was incubated with a cell monolayer in a T25 flask (target cells) at 4 °C for 45 min. Cells were washed twice with washing buffer, and then the bound RNA aptamers were eluted by heating at 95 °C for 5 min and separated by phenol:chloroform:isoamyl alcohol (Tripure, Roche) and chloroform extraction. The obtained DNA was polymerase chain reaction (PCR)-amplified with primers (25 cycles of 30 s at 94 °C, 30 s at 58 °C, and 30 s at 72 °C, followed by 5 min at 72 °C; the Taq polymerase and deoxynucleotide triphosphate (dNTP) were obtained from Promega. In the first round selection, the amount of initial ssDNA pool was 12 nmol, dissolved in 1 ml of binding buffer; and the counter selection step was eliminated. In order to acquire aptamers with high affinity and specificity, the wash strength was enhanced gradually by extending wash time (from 1 to 10 min), increasing the volume of wash buffer (from 1 to 5 ml) and the number of washes (from 3 to 5). Additionally, 20% FBS and 50–300 fold molar excess 45-mer random DNA library (a completely different library) were added to the incubation solution to reduce the nonspecific binding of the selected pool. After 20 rounds of selection, the selected ssDNA pool was PCR-amplified using unmodified primers.
2.3. Preparation of low-molecular-weight (MW) Chi
To obtain a low MW Chi, medium MW Chi was depolymerized according to the method described by Moghaddam et al [1]. Briefly, 1 g Chi (400 kDa) was dissolved in acetic acid (6% v/v). Low MW Chi was obtained after addition of 10 ml of NaNO2 (7.0 mg ml−1) to the dissolved Chi at room temperature under magnetic stirring. After 1 h, the depolymerized Chi was precipitated by raising the pH to 9.0 by adding NaOH (4 N). Centrifugation was 10 000 rpm for 5 min. The white yellowish solid was filtrated, washed with acetone three times, and dissolved in a minimum volume of 0.1 N acetic acid. Purification was carried out by subsequent dialysis at MW of 12–14 kDa against purified water (D-TubeTM Dialyzer Maxi, Merck Millipore, USA). The yellowish lyophilized product was then stored at 4 °C until further use.
2.4. Preparation of Chi-TPP-PF copolymer
The preparation of Chi NPs was achieved via the ionic-gelation method by Calvo et al [15]. A Chi solution (0.1% w/v) was obtained by dissolving low MW Chi in 1% v/v acetic acid. Chi NPs were prepared spontaneously upon addition of various concentrations of TPP (0.01%, 0.015%, 0.02%, 0.025%, and 0.03% w/v) to Chi solution under gentle magnetic stirring at room temperature for 1 h. In all cases, the volume ratio of Chi-TPP solution was 2:1. PF was incorporated into NPs by adding 0.025% w/v TPP solution to Chi aqueous solution containing different concentrations of PF (10%, 15%, 20% w/w).
2.5. Synthesis of carboxyl-terminated Chi-TPP-PF copolymer
A carboxylic acid group was introduced to the chain end of PEO-PPO-PEO (PF, MW = 12 500) by reaction of the terminal hydroxyl group of pluronic®F127 with succinic anhydride as described in the reference [16]. Briefly, Chi-TPP-PF copolymer (5 g, 0.75 mmol), succinic anhydride (0.56 g, 3 mmol), DMAP (0.5 g), and TEA (0.5 ml) were dissolved in 30 ml anhydrous 1,4-dioxane and stirred overnight at 30 °C. Then the 1,4-dioxane was removed under a centrifuge vacuum. The residue was dissolved in chloroform, precipitated into an excess of diethyl ether, and then filtered to remove un-reacted succinic anhydride, DMAP and TEA. With repeating the process twice, the precipitate, carboxyl-terminated pluronic (about 85% yields), was obtained after filtering and drying in a vacuum for 24 h.
2.6. Preparation of core–shell NPs
The procedure of the reaction between carboxyl-terminated Chi-TPP-PF copolymer and PTX was as follows: 4 g (0.7 mmol of –COOH groups) of carboxyl-terminated Chi-TPP-PF copolymer was dissolved in 2 ml of N, N-dimethylformamide (DMF) at room temperature. 0.1 g of EDC and 0.2 g of NHS was added in carboxyl-terminated Chi-TPP-PF copolymer solution. After 15 min reaction, 1.5 ml of 2-mercaptoethanol was added to quench the EDC followed by an addition various concentrations of PTX (0.2, 0.4, 0.6, 0.8 and 1.0 mg ml−1) to the activated carboxyl-terminated Chi-TPP-PF copolymer. The activated carboxyl-terminated Chi-TPP-PF copolymer in DMF was reacted with PTX in the presence of triethylamine (1 mg) and stirred at room temperature for 12 h under nitrogen. The resultant was dialysed against DMF by using a dialysis cassette with MWCO of 3.5kD for 24 h and finally lyophilized to gain the product.
2.7. Characterization of NPs
The morphological examinations of NPs were made by scanning electron microscopy (SEM) (440, Leica Cambridge Ltd, Cambridge, UK). NPs were dried on an aluminum disk at room temperature. The fixed NPs were coated with gold using a sputter coater (Desk II, Denton Vacuum, Moorestown, NJ).
Transmission electron microscopy (TEM) (JEM 1230, Joel Ltd, Tokyo, Japan) was used to examine and compare the topography of the NPs. Freeze dried NPs were suspended in milli Q water before observation.
2.8. Size measurements and determination of zeta potential
Size and zeta potential (surface charge) of NPs were measured at least in triplicate using nano partica SZ-100 (Horiba, Japan). For both of the above measurements, 0.2 mg NPs was suspended in 10 ml milli Q water.
2.9. In vitro drug release
PTX release was determined by incubating the NPs in PBS at 37 ± 0.5 °C. Remaining free PTX in the supernatant was measured for its absorbance at λ = 280 nm by using UV–vis spectrophotometer. For each sample, the release medium was withdrawn at predetermined time intervals at 12 d and replaced by the same medium at the same condition [3]
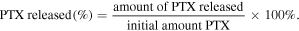
2.10. Evaluation of drug encapsulation and LC
0.5 mg NPs were dissolved in 5 ml milli Q water and centrifuging at 14 000 rpm for 30 min at 15 °C. The amount of PTX released in the supernatant was measured by spectrophotometry at 247–249 nm. Each sample was measured in triplicate. The following equations were used to evaluate the LC and encapsulation efficiency (EE) of the NPs [6]:
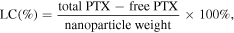
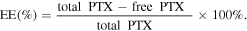
2.11. In vitro cytotoxicity
The cytotoxicity of PTX NPs and free PTX were performed on SK-BR-3 human breast cancer cell-line, NS-VN-67 colon cancer cells and HT-VN-26 stomach cancer cells of Vietnamese patients. The cell-lines were cultured in Mc'Coy 5A and DMEM supplemented with 10% FBS, L-glutamine, and 0.25 μg ml−1 ceftriaxon solution and incubated at 37 °C at 5% CO2. Cells were seeded at a density of 12 000 viable cells per well in 96-well tissue culture plates (Corning Incorporated Costar®) incubated for 24 h to allow cell attachment. The cells were then incubated for another 12, 24 and 48 h with PTX copolymer NPs and free PTX. Cells were then washed in PBS, and 20 μl of MTT solution (5 mg ml−1) was added to each well. The plates were incubated for an additional 4 h and then the medium was discarded. 200 μl DMSO was added to each well, and the solution was vigorously mixed to dissolve tetrazolium dye. The absorbance of each well was measured by PlateReader AF2200 (Eppendorf, Germany) at 570 nm.
2.12. Statistical analysis
Results are shown as mean ± standard deviation and each experiment was measured in triplicate. Statistical data analyzes were performed by applying one-way ANOVA tests and P-value <0.05 was considered significant.
3. Results
3.1. SELEX for enrichment of DNA aptamer candidates for target cells
To isolate aptamers with high affinity and specificity to the native, membrane presented form of HER-2, we performed SELEX using a well-known HER-2 overexpressing breast cancer cell line, SK-BR-3. We started the SELEX procedure by using a DNA library of 45 nt randomized region, with 1014 complexity. We performed positive selection by retrieving aptamers that bind to SK-BR-3 cells. After 20 rounds of positive selection, the binding affinity of DNA library reached saturation (data not shown). After the successful enrichment of aptamers with high affinity to SK-BR-3 cells, individual aptamers were sequenced (table 1).
Table 1. All of the oligonucleotides used in this work.
| Name | Sequence |
|---|---|
| Library | 5'-TCA CCG GGA GGA GAC CCT GA-N40-GTG GCT TGG TGG TGG TTC AA -3' |
| Aptamer | 5'-AAC TTG GTG GTG GTT CGG TGG CTG TTC AGG GTC TCC TCC CGG TGA-3' |
N is base number which is equal to the corresponding aptamer sequences.
3.2. Preparation of Chi-TPP NPs
The NPs were prepared by ionic-gelation upon the addition of TPP to Chi in acetic acid under gentle magnetic stirring at room temperature. The NPs with smaller size have valuable characteristics such as more improved drug delivery, longer circulation in blood, and lower toxicity. The conditions of experiments were carried out at the concentrations of low MW Chi 1% (v/v) in acetic acid and TPP 0.01%, 0.015%, 0.02%, 0.025% and 0.03% (table 2).
Table 2. Effect of TPP concentration on mean diameter and zeta potential of Chi NPs.
| TPP concentration (%w/v) | Mean diameter (nm) | Zeta potential (mV) |
|---|---|---|
| 0.010 | 26.24 ± 4.37 | 49.18 ± 1.14 |
| 0.015 | 29.43 ± 1.23 | 43.22 ± 0.58 |
| 0.020 | 37.11 ± 5.42 | 40.31 ± 1.52 |
| 0.025 | 53.26 ± 3.21 | 38.30 ± 0.92 |
| 0.030 | 82.51 ± 7.46 | 35.82 ± 0.34 |
Due to dissociation, TPP dissociation in water and hydroxyl ions liberated, the TPP solution exists and the OH− ions and ion  had been competitive reaction with NH3+ group of Chi. The low MW Chi fragments were participated in the reaction with TPP molecules ionic-gelation, combined with the agitation to form smaller size Chi NPs.
had been competitive reaction with NH3+ group of Chi. The low MW Chi fragments were participated in the reaction with TPP molecules ionic-gelation, combined with the agitation to form smaller size Chi NPs.
Chi NPs did not generate delamination after 3 d stored in 4 °C, suggesting that NPs formed had been smaller if the larger NP size would have been caused sedimentation and phase separation during storage.
SEM images showed that the shape of Chi original material each large polymer particle size. Chi NPs generated by ionic-gelation method using TPP, with small particle size, relatively uniform (figure 1).
Figure 1. SEM image of Chi: (a) Chi before ionic-gelation and (b) Chi NPs generated by ionic-gelation method using TPP with ratio 2:1.
Download figure:
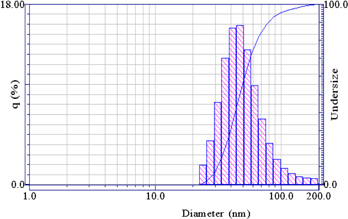
Standard image High-resolution imageThe results of the particle size distribution showed that the average was 53.2 nm. They were included about 81% particles size of 55.6 nm; 9% ones of 38 nm and larger than 100 nm (figure 2).
Figure 2. Particle size and distribution of Chi NPs.
Download figure:
Standard image High-resolution image3.3. Preparation of Chi-TPP-PF NPs
The addition results of pluronic®F127 (PF) in decreased size which could be attributed to the formation of a rigid gel leading to lesser water uptake. Slighter swelling caused a reduction in the mean diameter of the particles in aqueous medium (table 2). Since there was not significant change in zeta potential by increasing the concentrations of PF (P < 0.028), it could be concluded that PF was mostly incorporated inside the NP matrix, the Chi 1%-TPP 0.025% NPs were mixed in different concentrations of PF (table 3).
Table 3. Effect of PF concentration on mean diameter and zeta potential of Chi 1% NPs.
| PF (%w/v) | Mean diameter (nm) | Zeta potential (mV) |
|---|---|---|
| 10 | 61.24 ± 3.17 | 32.14 ± 0.36 |
| 15 | 69.11 ± 6.42 | 28.47 ± 1.42 |
| 20 | 64.53 ± 1.42 | 30.14 ± 0.91 |
3.4. Characterization of the conjugation of aptamer to PF
Aptamer modified pluronic ®F127 (PF-Ap) was synthesized by the reaction of carboxylated pluronic ®F127 with the amino groups at the ends of aptamers (figure 3(a)). PF-Ap containing a scrambled aptamer was obtained by the same method. To confirm the conjugation and determine the maximum conjugation content of Ap on pluronic ®F127, novex®12% tris-glycine mini gel electrophoresis was carried out. The samples including free Ap, Chi-TPP-PF, and Chi-TPP-PF-Ap with or without EDC-NHS used in the conjugation reaction were separately subjected to 2% tris borate edta sodium dodecyl sulfide polyacrylamine gel electrophoresis (TBE SDS PAGE). Then the electrophoresis was performed at 125 V for 30 min, and the base pair band on the gel was displayed by SYBR®green nucleic acid stain (figure 3(b)). The detailed synthesis procedure, chemical compositions, and characterization of the materials were given in supplementary information.
Figure 3. Synthesis and confirmation of polymers. (a) Synthesis route of pluronic®F127-Ap, (b) determination of the conjugation of aptamer to pluronic®F127 via novex®12% tris-glycine mini gel electrophoresis.
Download figure:
Standard image High-resolution image3.5. Nanoparticle EE and LC drug
The general addition of PTX increased the sizes of Chi NPs, but did not affect their zeta potential significantly (P < 0.02). The effect of PTX concentration on particle size is more significant than when the concentration rises 0.2–0.4 mg ml−1 (P < 0.015). The PTX-loaded Chi-PF NPs size did grow significantly at concentrations up to 0.6–1 mg ml, but there was a change in size when the concentration of PTX 0.4 mg ml were increased maximum (EE = 83.28 ± 0.13%; LC = 9.12 ± 0.34%) and mean diameter 86.22 ± 1.45 nm. PTX was a low MW anti-cancer drug. Therefore, it might not be possible to increase the PTX particle diameter severely until it reached its maximum capacity inside the NPs. The PTX concentration did not influence the zeta potential of the prepared NPs (P < 0.04), Chi 1%-TPP 0.025%-PF 15% NPs were loaded in different concentrations of PTX (table 4).
Table 4. Characterization, encapsulation efficiency and loading capacity of PTX encapsulated core–shell NPs.
| PTX (mg ml−1) | Mean diameter (nm) | Zeta potential (mV) | EE (%) | LC (%) |
|---|---|---|---|---|
| 0.2 | 65.11 ± 3.81 | 23.22 ± 1.56 | 67.31 ± 0.51 | 3.94 ± 1.19 |
| 0.4 | 86.22 ± 1.45 | 60.12 ± 0.55 | 83.28 ± 0.13 | 9.12 ± 0.34 |
| 0.6 | 89.55 ± 2.05 | 48.35 ± 0.24 | 68.01 ± 3.77 | 8.15 ± 3.63 |
| 0.8 | 99.32 ± 7.14 | 40.21 ± 1.43 | 80.62 ± 1.28 | 6.41 ± 1.23 |
| 1 | 110.46 ± 4.32 | 38.33 ± 1.12 | 59.36 ± 1.53 | 5.85 ± 1.57 |
3.6. Morphology of NPs
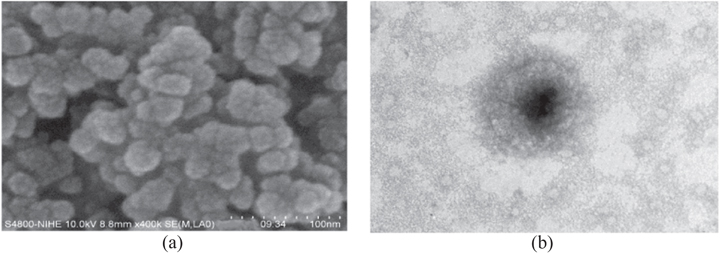
SEM and TEM images of Chi-PF NPs loaded with PTX, showed that particles were spherical and uniform with mean diameter 86.22 nm (figure 4).
Figure 4. (a) SEM and (b) TEM images of Chi 1%-TPP 0.025%-PF 15% NPs loading PTX 0.4 mg ml−1.
Download figure:
Standard image High-resolution image3.7. In vitro drug release of PTX
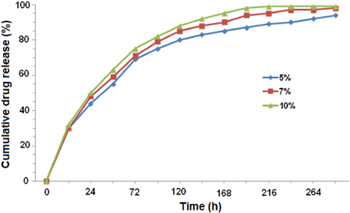
The amount of released PTX was presented as the percentage of cumulative release at 37 °C over a period of 12 d. The drug concentration was determined by a fluorescence spectrophotometer at excitation wavelength of 280 nm. Nanoparticle solution was used for the experiments with concentrations of 5%, 7%, and 10%. In the first 12 h, followed by sustained release of 29%–35% and 85%–93% after 288 h at pH 7.5. This result suggested that there were two phases of PTX release profile. Firstly, the initial burst release of the PTX from the NPs in the first 12 h. Burst release was the phenomenon of drug which a greater amount of initial bulky drug were immediately released prior to arriving at the steady level of the release profile. This direction affected the effective exposure time of nano-carriers. In the next phase, a sustained release of the encapsulated PTX was shown after the 6th day, Chi 1%-TPP 0.025%-PF 15% NPs were loaded with PTX 0.4 mg ml−1 (figure 5).
Figure 5. PTX release profiles in PBS at pH of 7.5.
Download figure:
Standard image High-resolution image3.8. In vitro cytotoxicity
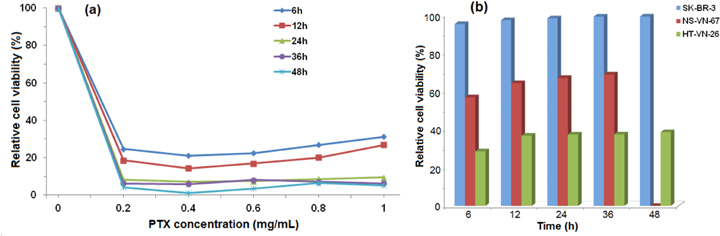
Cytotoxicity test was performed on the NPs without PTX, Chi 1%-TPP 0.025%-PF 15% NPs loaded with PTX at concentrations of 0.2, 0.4, 0.6, 0.8 and 1 mg ml−1, using the MTT method on SK-BR-3 cells. The NPs without PTX were shown to have minimum toxic effects and themselves would not cause cellular damage. Their half maximum inhibitory concentration (IC50) is 7.3–9.5 mg ml−1 (figure 6(a)).
Figure 6. Cell viability of (a) SK-BR-3 versus different concentrations of PTX and (b) SK-BR-3, NS-VN-67, HT-VN-26 with Ap-micelle-PTX at different times.
Download figure:
Standard image High-resolution imageThe toxic cell death of NPs without PTX did not change much after 6, 12, 24, 36 and 48 h. Living cell proliferation did grow up grip bottom flask. Therefore, it could be concluded that the incorporation of PF was not influence the cytotoxicity of the NPs. The effect of free PTX 1 mg ml−1 solution on cell death was also investigated. The relative cell viability after 6, 12, 24, 36 and 48 h was 86.5%, 72.77%, 65.82%, 32.8% and 22.55%, respectively.
The IC50 of free PTX against SK-BR-3 cell was 1 mg ml−1 which was about five times lower than NPs loaded PTX. On the other hand, the NPs non-loaded PTX processing lyophilized drug stored when reconstituted in the milli Q water, they were not toxic to cells.
With the effect of NPs Chi 1%-TPP 0.025%-PF 15%-PTX 0.4 mg ml−1, the SK-BR-3, NS-VN-67 and HT-VN-26 were dead 91%–95%, 52%–56% and 26%–28%, respectively, after 6 h. The partition was destroyed and led to broken cells after 6 h. PTX was a hydrophilic anti-cancer drug that needed membrane transporters to enter the cells. Cells could uptake NPs by endocytosis mediated by a clathrin-mediated process. Therefore, the NPs could act as drug delivery systems that facilitated drug entrance into the cells. With the effect of free PTX 1 mg ml−1 the cell dead proportion was 13%–14%. It did not affect the cells efficiently. The above-mentioned results of the in vitro cytotoxicity test showed that the NPs were biocompatible and used as anti-cancer drug carriers.
4. Conclusions
In this study, using SELEX, we isolated DNA aptamer which can bind with high affinity and specificity to HER-2. Chi-TPP-PF-Ap was used to synthesize anew aptamer-pluronic®F127 conjugate. A nanoparticulate formulation of PTX was formulated successfully. The drug loaded Ap-micelles showed higher cytotoxicity which compared to blank Ap-micelles and free drug. The Ap-micelles loaded PTX were spherical and uniform with mean diameter 86.22 nm and zeta potential 60.12 mV. EE and LC were 83.28% and 9.12%, respectively. The amount of released PTX was profile at pH 7.5 over 12 d. The PTX-Ap-micelles were more cytotoxic against SK-BR-3 cells than was free PTX about five times. We successfully developed a novel aptamer micelle assembly for efficient detection and delivery system for PTX targeting specific breast cancer cells. This aptamer micelle enhances the binding ability of the aptamer moiety at physiological temperature, even though the corresponding free aptamer loses its binding ability under the same condition. All of these advantages endow this unique assembly with the capacity to function as an efficient detection and delivery vehicle in the biological living system.
Acknowledgements
This work was supported by State Program 'Application-oriented basic scientific research' Project number 04/2011/HD-NCCBUD.