Abstract
In recent years researchers were attracted towards marine sources due to the presence of active components in it. Seaweeds were widely used in pharmaceutical research for their known biological activities. The biological synthesis method of silver nanoparticles (AgNPs) using Padina tetrastromatica seaweed extract and their cytotoxicity against breast cancer MCF-7 cells was reported in this study. The synthesized AgNPs using seaweed extract were subjected to x-ray diffraction, UV–visible spectroscopy, Fourier transform infrared spectroscopy, field emission scanning electron microscopy, transmission electron microscope, energy dispersive x-ray, zeta potential to elucidate the structural, morphology, size as well as surface potential parameters. An absorption peak at 430 nm in UV-visible spectrum reveals the excitation and surface plasmon resonance of AgNPs. FE-SEM micrographs exhibits the biosynthesized AgNPs, which are pre-dominantly round shaped and the size ranges between 40–50 nm. The zeta potential value of −27.6 mV confirms the stable nature of biosynthesized silver nanoparticles. Furthermore, the biological synthesized Ag NPs exhibited a dose-dependent cytotoxicity against human breast cancer cell (MCF-7) and the inhibitory concentration (IC50) was found for AgNPs against MCF-7 at 24 h incubation. Biological method of synthesizing silver nanoparticles shows a environmental friendly property which helps in effective electrifying usage in many fields.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Many products derived from algae have high demand in biological research due to its abundant richness of bioactive compounds [1]. In recent trends, the process of combining of green chemistry principles to nanotechnology is one of the important challenges of research at nanoscale. Usually ranging from 1 to 100 nm, the application of nano sized products and structure is a rising area of nanoscience and nanotechnology. The synthesis of size tunable nonmaterial has many applications in various field such as biomedical [2], pharmaceutical, cosmetic, environmental, and electronics [3]. Silver nanoparticles (AgNPs) have been extensively applied in surgical instruments, bond prostheses, wound dressings, electronics and biosensing [4, 5]. AgNPs can catalyze many reduction reactions. They can also act as a good catalyst [6]. In brown algae, the sulphated polysaccharides are polyanionic in nature and they can bind to charged molecules, which is positive through poly electrolyte complextion. Hence, they are widely used for nanomaterials synsthesis and nanobiomedical research that includes the synthesis of nanomaterials for drug delivery purposes [7].
Padina tetrastromatica is the family of Dictyotaceae, which is one of the most admissible marine brown seaweeds. It contains the major component of fucopyranosyl residues containing sulfated fucans called as fucoidans, alginc acid, glucans minor amount of glucronic acid and uronic acid are also found. They have an important role in the field of antiviral activities [8]. Over the past 40 years, many marine organisms have been reported for their anti-tumor cytotoxic substances [9]. Marine seaweeds and fungi found to have anti bacterial activities [10]. Many compounds have also been isolated from marine sources. Such substances are playing very promising role in cancer research. Numerous clinical trials were made to screen the property of marine source based compounds as anti-tumor agents. Compounds like polysaccharides and terpenoids from red and brown algae are considered as efficient bioactive substances with anticancer activity [11]. Fucoxanthin extracted from marine algae has found to reduce the number of tumor nodules on the surface of lung [12]. In the past decade, several kinds of the biological organisms like seaweeds, plants and microbes have been well studied for the synthesis of NPs [13]. The biomolecules extracted from the algae Padina tetrastromatica having a lot of applications in the field of medical biochemistry. Ascophyllan compound from Padina tetrastromatica was found to have highest antioxidant activity [14]. Polysaccharides such as dietary fibers, alginates, mannitol, agar, laminarian are found to be economically important chemicals used in food and cosmetic industries [15]. Seaweed coated with starch biopolymer showed cell death in breast cancer cells. Phenolic compounds present in seaweed also show excellent anti-cancer activity [16]. However, among the other brown algae, Padina tetrastromatica got very low attention in terms of biomedical research. Hence, in the present study, an effort is made to biosynthesize AgNPs using the seaweed extract of Padina tetrastromatica. Thereafter, the cytotoxic effect of AgNPs against MCF-7 breast cancer cell line has also been evaluated.
2. Materials and methods
2.1. Collection of seaweed
The marine algae Padina tetrastromatica were collected from Mandapam seacoast, Ramanathapuram district, Tamilnadu. The collected samples were rinsed in seawater to remove extraneous materials and washed with distilled water. The samples were brought to the laboratory in sterile polythene bags. The collected seaweed species was identified taxonomically as Padina tetrastromatica and then used for further analysis. Thereafter, the seaweeds were shade dried and blended in electric blender and stored at 4 °C for further use.
2.2. Biosynthesis of AgNPs
For biosynthesis of AgNPs, 1 g of seaweed powder was extracted with distilled water and filtered. Green synthesis of AgNPs was carried out by the following method [17]. To 1 mM silver nitrate solution, 10 ml of aqueous Padina tetrastromatica extract was added and continuously stirred using magnetic stirrer for 72 h at 37 °C. The reduction of Ag+ ions changes a green color solution into yellowish brown color solution which indicates the formation of AgNPs.
2.3. Characterization of AgNPs
The biosynthesized AgNPs were examined for its optical properties using 10 mm optical path-length quartz cuvettes with a Shimaduz, UV–vis range 3600 spectrophotometer. A Perkin-Elmer FTIR spectrum-RX-1 spectrophotometer was used to record the functional groups at a resolution of 2 cm−1 ranging from 4000 to 400 cm−1 with KBr pellets in diffuse reflectance mode. A drop of colloidal NPs were coated on a copper plate, it was dried using hot air and investigated under field emission scanning electron microscopy (FE-SEM Hitachi SU6600) to characterize the shape and size of AgNPs. The structure of the synthesized AgNPs was viewed under high resolution transmission electron microscope. 3 μl of the colloidal AgNPs was placed on the copper grid and allowed to dry for 15 min at room temperature. The structural image of the biosynthesized silver nanoparticles were obtained on an EM TECNAI microscope operated at 200 kV equipped with energy dispersive x-ray (EDX) spectra. A thin film glass substrate with a Philips 86 x'pert pro x-ray diffractometer was used to measure the XRD pattern of the synthesized AgNPs. Dynamic light scattering analysis was also performed to examine the average size distribution as well as the stability of the synthesized AgNPs using zeta sizer Nano ZS90 (Malvern, UK).
2.4. Cytotoxicity of biosynthesized AgNPs
Breast cancer cell line MCF-7 were obtained from National Centre for Cell Science (NCCS), Pune, and cultured in Dulbeco's modified eagle medium (DMEM), supplemented with essential aminoacids at 37 °C in a humidified CO2 incubator [18]. Cells were seeded onto the plates at a density of 1 × 105 cells/well. After incubation, the cells were washed with 100 μl of serum-free medium and allowed to starve at 37 °C. After starvation, the cells were again treated with different concentrations of biologically synthesized AgNPs (10–200 μg ml−1) for 1 day. Incubated cells were then subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, after incubation the cells were washed with 200 μl of phosphate-buffered saline (PBS) solution. The resulting formazon was dissolved by adding 100 μl of dimethyl sulfoxide (DMSO) by gentle pipettting up and down and the absorbance of the purple blue formazan dye was measured in a microplate reader at 570 nm (Biorad 680). Cytotoxicity was determined using graph pad prism5 software. The obtained optical density (OD) value was subjected to sort out the percentage of viability by using the following formula
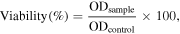
where ODsample is mean OD value of experimental sample (AgNPs), ODcontrol is mean OD value of experimental control (untreated).
2.5. Morphological observations
Breast cancer cell lines MCF-7 (1 × 105 cells) were grown in a six-well plate. It was incubated with AgNPs at different concentrations for 24 h. After the desired incubation period both dead and live cells were collected and centrifuged at 4000 rpm for 20 min. The pellet was collected and resuspended with phosphate buffer solution and stained with AO/EB (1:1 ratio) [19]. After the incubation period with the AgNPs, the suspension was immediately examined under a Nikon inverted fluorescent microscope (Ti-eclipse) at 400× magnifications and the untreated cells served as control. A minimum of 300 cells was counted in each sample. Results were expressed as means ± SE for three independent determinations.
2.6. DNA laddering
The cells were harvested after 24 and centrifuged at 10 000 rpm for 10 min at 4 °C. The cells were lysed in lysis buffer (10 mM Tris-cl, pH 7.5, 1 mM ethylenediaminetetraacetic acid (EDTA) and 0.2% triton x-100) for 30 min. The lysate was incubated with DNA extraction buffer (0.1 mg ml−1 proteinase K and 0.2 mg ml−1 RNase) for 1 h at 60 °C. Fragmented DNA was extracted with phenol/chloroform (1:1) and run in 1% agarose gel containing 2% ethidium bromide. The lanes 2, 3, 4 and 5 were loaded with control cells and the colloidal silver NPs of 50 μg ml−1, 100 μg ml−1 and 200 μg ml−1, respectively. The lane 1 was loaded with marker (100 bp-invitrogen). The DNA fragments were viewed by exposing the gel to UV-transilluminator and photographed.
2.7. Caspase activity assay
Cells were seeded in 12-well plates, treated with different concentrations of Ag NPs with 10% PBS-DMEM. Caspase -3 activity in MCF-7 cells was performed using a colorimetric caspase-3 assay kit. The assay is based on the principle that activated caspases in apoptotic cells cleave the peptide substrates to release free chromophore pNA (short caspase specific peptides). Approximately 5 × 104 cells were used for each sample. The sample concentration was used from range of 0, 50, 100 and 200 μg ml−1. The apoptotic cells were estimated by elevated level of intracellular compared with and without compound treated cells. The absorbance of the cleaved chromogenic peptide substrates was measured using the scan II plate reader (Biorad, USA) at 405 nm. Chromogenic units were converted to percentage by comparing the untreated control cells generated free pNA. Quantification of caspase activity was calculated as fold increase over control samples.
3. Results and discussion
3.1. UV–vis absorption studies
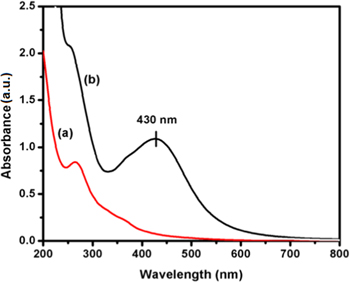
The use of biological source in the biosynthesis of AgNPs and its characterization are important in nano biomedical research. The formation of AgNPs is confirmed by the change in colour, where as there was no color change in control. Silver nitrate (1 mM) was used as control. The UV–vis absorption spectrum of seaweed Padina tetrastromatica extract and biosynthesized AgNPs are shown in figure 1. The maximum absorbance at 264 nm was observed for the seaweed Padina tetrastromatica extract, when the addition of seaweed extract with colourless solution of AgNO3 was changed into yellowish brown. The change in colour may be due to the excitation of surface Plasmon resonance and thus indicates that the occurrence of Ag+ reduction to AgNPs [20, 21]. The free electrons of AgNPs give rise to a surface plasmon resonance absorbance due to the combined vibration of electrons of the metal NPs in resonance with the light waves [22]. The observed λmax value at 430 nm in the UV–vis spectrum confirms that the formation of AgNPs.
Figure 1. UV–vis absorbance spectrum of (a) seaweed Padina tetrastromatica extract, and (b) biosynthesized AgNPs using seaweed Padina tetrastromatica extract.
Download figure:
Standard image High-resolution image3.2. X-ray diffraction studies
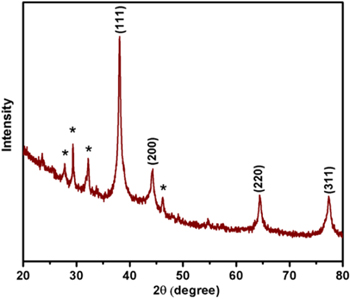
The x-ray diffraction (XRD) peaks of AgNPs exhibited at 2θ = 38.1°, 44.3°, 64.3° and 77.3°, which are corresponds to the (111), (200), (220) and (311) diffraction planes of silver. The AgNPs crystallizes in face-centered cubic structure and the obtained results were in good agreement with the diffraction planes of silver JCPDS No. 04-0783. The crystallite size (d) of the biosynthesized AgNPs was determined using Scherrer's equation [23], d = 0.9λ/Bcosθ, where λ is the wavelength of x-ray radiation, B is the full width half maximum value in radian and θ is the Bragg's diffraction angle. The estimated crystalline size using Scherrer's formula for the biosynthesized AgNPs was found to be 35.2 nm. Some of the other peaks which are presented in the XRD pattern of AgNPs, may be due to crystallization of bio-organic phase [22]. The XRD pattern of biosynthesized AgNPs is shown in figure 2.
Figure 2. XRD pattern of biosynthesized AgNPs using Padina tetrastromatica extract.
Download figure:
Standard image High-resolution image3.3. FTIR studies
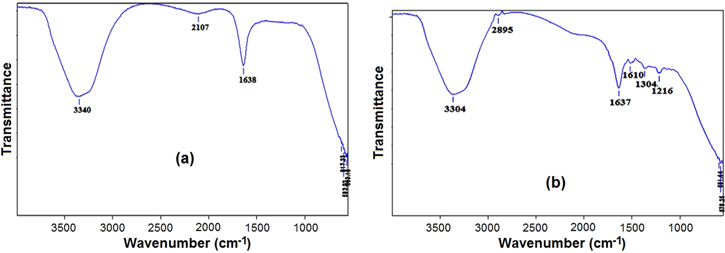
In the FTIR spectrum of seaweed extract, the strong absorption bands at 3340 cm−1 and 1638 cm−1 are corresponds to the O-H and C=O stretching vibration modes of hydroxyl group and a amide group, respectively [24, 25]. The carbonyl (C=O) modes in the amide groups was involved in the reduction of Ag+ to Ag0. In the spectrum of AgNPs, the absorption peak at 3304 and 1637 cm−1 are attributed due to the hydroxyl group and binding of C=O functional group with AgNPs [26]. The observed two minor peaks at 2895 and 1304 cm−1 corresponds to the stretching and bending vibration of C-H alkane group. The peak at 1618 cm−1 indicates to the stretching vibration of (NH) C=O group [27]. The FTIR results suggest that the presence of functional group in the extract serve as the reducing and capping agent of the AgNPs (figure 3).
Figure 3. FTIR spectrum of (a) Padina tetrastromatica extract and (b) biosynthesized AgNPs.
Download figure:
Standard image High-resolution image3.4. Particle size distribution and zeta potential studies
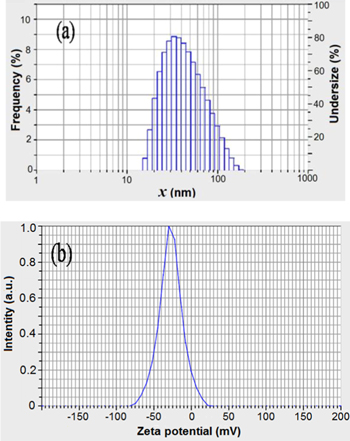
Size distribution analysis was performed using dynamic light scattering to know the size of the biosynthesized silver nanoparticles. Dynamic light scattering results affirmed an average diameter of the biosynthesized AgNPs was in nanometer range. The intensity weighed particle size distribution histograms are shown in figure 4(a). The obtained AgNPs exhibited polydisperse mixture with the size ranging of 43.1 nm with poly dispersity index of 1.017. Thus, the particle size distribution studies confirm that the Ag particle has formed in nanosize and well match with the average particle diameter range obtained from FE-SEM studies.
Figure 4. (a) Particle size distribution, and (b) zeta potential measurement of the biosynthesized AgNPs using Padina tetrastromatica extract.
Download figure:
Standard image High-resolution imageZeta potential is the net surface charge of NPs. The parameter studies used to determine the stability of AgNPs, which shows the electrostatic potential at the electrical double layer surrounding a nanoparticle in solution. The zeta potential distribution plot is shown in figure 4(b). The stability of NPs depends on the zeta potential value and the value should be either a large negative or positive (∼ −30 mV or +30 mV). The obtained zeta potential −27.6 mV indicates that they are strongly anionic. In biological applications it is proven that colloidal silver (strongly anionic) has important application [21]. However, since cellular membranes are negatively charged the efficiency of zeta potential is important for permeation. Here the biosynthesized AgNPs showed that they are strongly anionic, suitable for biological application.
3.5. FE-SEM studies
The FE-SEM images of biosynthesized AgNPs using seaweed padina tetrastromatica extract is shown in figure 5. The micrograph of synthesized AgNPs showed the uniform spherical in shape with a average diameter range of 40–50 nm.
Figure 5. FE-SEM images of biosynthesized AgNPs using Padina tetrastromatica extract.
Download figure:
Standard image High-resolution image3.6. TEM studies
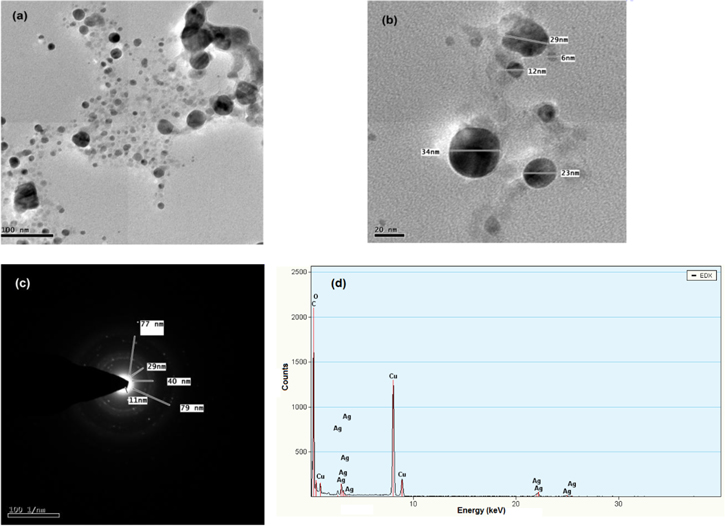
The morphology of the particles was imaged using high-resolution transmission electron microscopy (HRTEM). The sample was processed by dropping 3 μl of the sample on the copper grid followed by drying at room temperature for 15 min. The average mean size of the AgNPs was around 6–40 nm (figures 6(a) and (b)).
Figure 6. (a) and (b) HRTEM images, (c) SAED pattern and (d) EDX pattern of biosynthesized AgNPs.
Download figure:
Standard image High-resolution imageThe shapes of the NPs are spherical in shape. The elemental analysis of the biosynthesized silver nanoparticles was confirmed using the EDX on the TEM. The spectrometers confirmed the presence of elemental silver signal of the AgNPs (figure 6(d)). The number of x-ray counts were displayed in the vertical axis (500–2500), the energy in keV was represented in the horizontal axis. The peaks around 4 keV, 23 keV and 25 kev corresponds to the binding energies of silver, thus giving confidence that only silver is formed without impurities. Along with silver peaks, a single copper peak also observed, this is be due to the copper grid. The selected area electron diffraction (SAED) pattern shows the crystalline nature of NPs that agrees with crystalline and face centered cubic structure of AgNPs. The sharp spots in the SAED pattern indicate that the AgNPs are single crystalline in nature (figure 6(c)).
3.7. Cytotoxicity studies
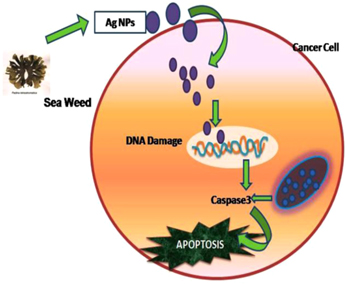
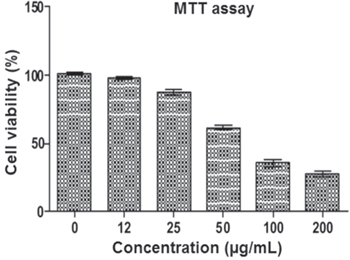
In medical applications, there is a greater demand for AgNPs as an antimicrobial agent. Only limited reports are present in the cytotoxic studies of seaweed mediated synthesized AgNPs against cancer cell line [16]. Many marine organisms like Celina radiata has the capability of inhibiting cancer cells [28]. The possible mechanism of the biosynthesized AgNPs against cancer cell line is shown in figure 7. The invitro cytotoxicity of biologically synthesized AgNPs was investigated by an MTT assay against MCF-7 breast cancer cell line with different concentrations like 12 μg ml−1, 25 μg ml−1, 50 μg ml−1, 100 μg ml−1and 200 μg ml−1, respectively (figure 8). Biologically synthesized AgNPs showed cytotoxicity on the MCF-7 cell line with increased concentration. The inhibitory concentration (IC50) value of biosynthesized AgNPs against MCF-7 cells was observed at 86.7 μg ml−1 in 24 h. When the cancer cells were treated with high concentration of AgNPs 200 μg ml−1, it exhibits high percentage of inhibition. Biologically synthesized AgNPs showed high inhibitory effect against cancer cell lines based on the increasing concentration was reported in few studies [27].
Figure 7. Possible mechanism involved in caspase mediated apoptosis of MCF-7 breast cancer cells induced by AgNPs of seaweed origin.
Download figure:
Standard image High-resolution imageFigure 8. MTT assay results confirming the in vitro cytotoxicity effect of AgNPs against the MCF-7 for 24 h. Data were expressed as mean ± SD of three experiments.
Download figure:
Standard image High-resolution image3.8. AO/EtBr staining
The ability of the AgNPs to induce apoptosis was determined by acridine orange (AO) and ethidium bromide (EtBr) staining. The morphological assessment of MCF-7 cancer cell lines was studied for different concentration 50 μg ml−1, 100 μg ml−1 and 200 μg ml−1 (figures 9(b)–(d)). Acridine orange and ethidium bromide staining reveals the morphological changes of the cancer cell lines after treating with different concentrations of silver nanoparticles. The stained cells were characterized to early apoptic (bright green fluorescence) late apoptotic cell (reddish orange fluorescence). Microscopic evaluations of the acridine orange and ethidium bromide stained cells revealed a dose dependent cell death and alteration in cellular morphology [29]. In the present study, few apoptotic bodies were seen in 50 μg ml−1 treated cancer cells (figure 9(f)). As the concentration of the biologically synthesized NPs was increased to 100 μg ml−1 more apoptotic bodies were seen as orange bodies as shown in figure 9(g). In 200 μg ml−1 concentration, shrinkage and blebbing of cells were observed along with it more apoptotic bodies were observed as orange and red coloured bodies (figure 9(h)). The control cells emits only green fluorescence, no orange bodies were seen (figure 9(e)).
Figure 9. (from (a) to (d)) Morphological assessment: (a) control, (b) 50 μg ml−1, (c) 100 μg ml−1, (d) 200 μg ml−1, and (from (e) to (f)) AO/EtBr staining: (e) control, (f) 50 μg ml−1, (g) 100 μg ml−1, (h) 200 μg ml−1.
Download figure:
Standard image High-resolution image3.9. DNA fragmentation
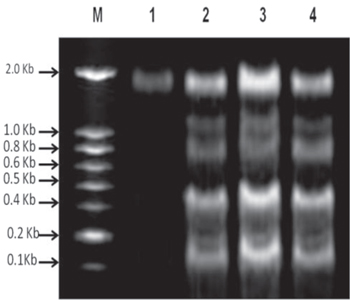
The MCF-7 cells treated with AgNPs induce the apoptosis through DNA damage was confirmed in DNA fragmentation assay. The induction of DNA single strand break is used to predict the damage of cancer cells [30]. In the present study, the AgNPs treated cancer cells showed apoptotic fragmentation DNA. The first lane treated with control cells doesn't show any fragmentation. The second lane treated with 50 μg ml−1 doesn't show any event. The third lane which treated with 100 μg ml−1 concentration induced a very low fragmentation, whereas 200 μg ml−1 showed moderate apoptotic fragmentation in figure 10.
Figure 10. M-100 bp DNA ladder (1) control cell DNA (2) 50 μg ml−1 treated cell DNA (3)100 μg ml−1 treated cell DNA, and (4) 200 μg ml−1 treated cell DNA.
Download figure:
Standard image High-resolution image3.10. Caspase-3 activity
Caspases are proteolytic, aspartate-specific cysteine protease enzymes which plays a key role in the apoptotic pathway of the cells [31]. To investigate the potential effect of biosynthesized silver nanoparticles on apoptotic pathway, the activity of caspase-3 was examined. A colorimetric assay kit was used to quantify the caspase-3 activity. In the present study, the increased level of caspase-3 activation suggested the AgNPs induced cell death through apoptosis. Quantification of caspase activity was calculated as fold increase over control samples (figure 11). The biosynthesized silver nanoparticle shows an increased concentration dependent activation of caspase-3. It shows the involvement of caspase-3 in the apoptotic event.
Figure 11. Effect of AgNPs on caspase-3.
Download figure:
Standard image High-resolution image4. Conclusion
In conclusion biosynthesization of AgNPs from Padina tetrastromatica shows promising results for biomedical applications. An absorption peak at 430 nm in UV–vis spectrum proven the formation of AgNP from Padina tetrastromatica. The zeta potential value of −27.6 mV revealed the stability of AgNPs. The results shown by high throughput techniques like UV-visible spectroscopy, FTIR, FE-SEM, XRD and zeta potential measurements indicate the successful formation of AgNPs. The present investigation suggests that the biosynthesized AgNPs are exerting cytotoxic effect on human breast cancer cell line MCF-7. However this in vitro cytotoxicity effect on MCF-7 breast cancer cell line proved the potential application of AgNPs synthesized from the seaweed Padina tetrastromatica. This AgNP involved in caspase-3 mediated apoptotic cell death.