Abstract
Breast cancer is one of the leading causes of deaths in females worldwide. The high metastatic rate and drug resistance makes it one of the difficult cancers to treat. Early diagnosis and treatment are keys to better survival of breast cancer patients. Conventional treatment approaches like chemotherapy, radiotherapy and surgery suffer from major drawbacks. Novel approaches to improve cancer therapy with minimal damage to normal tissues and better quality of life for cancer patients need to be developed. Among various approaches used for treatment and diagnosis of breast cancer, use of nanoparticles (NPs) is coming up as a new and promising treatment regime. It can help overcome various limitations of conventional therapies like non-targeted effects, resistance to treatment, late diagnosis, etc. Among various nanoparticles studied for their biomedical applications, especially for breast cancer therapy, iron oxide nanoparticles (IONPs) are perhaps the most exciting due to their biocompatibility, biodegradability, size and properties like superparamagnetism. Besides, IONPs are also the only metal oxide nanoparticles approved for clinical use in magnetic resonance imaging (MRI) which is an added advantage for early detection. Therefore in this mini review, we are discussing the developments made in the use of IONPs for breast cancer therapy over the short span of the last five years i.e. 2010−2015. Since late diagnosis and therapy resistance are important drawbacks in breast cancer therapy, the potential of IONPs to overcome these limitations are also evaluated.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Global cancer statistics show that in spite of many new and innovative strategies developed for effective detection and treatment of various cancers, the incidence of cancer has increased worldwide. Among various cancers diagnosed and treated, breast cancer is the 5th leading cause for cancer deaths. In India, breast cancer is the most commonly diagnosed cancer (21%) and one of the fatal cancers (21%). These figures are expected to increase in the future, thereby increasing the burden of this dreadful disease further [1].
Recently, the use of nanoparticles (NPs) in cancer therapy is gaining popularity. NPs because of their small size (1–100 nm) approach quantum dimensions resulting in change in internal energies and other properties. Therefore they exhibit unique optical, electronic, photochemical, magnetic and chemical properties which can be utilized variously for application in cancer therapy [2–6]. When a bulk material is made into NPs, there is a large increase in surface area and subsequently, a large number of surface atoms are exposed to interactions with other molecules. They are therefore more reactive than at the macroscopic scale. NP surfaces can be attached with various molecules like chemical compounds, drugs and proteins by covalent bonds or by absorption. This helps in targeted delivery of NP-tagged molecule/complexes to cancer tissues [7]. Passive targeting to cancer tissues can also be achieved due to their small size and enhanced permeability and retention (EPR) effect [8].
Thus contrary to conventional and clinically approved approaches to cancer therapy, NPs can specifically target (passively or actively) tumors resulting in reduced/little side effects and toxicities. They can also bypass p-glycoprotein associated drug resistance mechanism leading to better efficacy [9].
Therefore NPs are being explored for their use as therapeutic, diagnostic and thernostic (diagnosis and therapy at the same time) agents in cancer therapy [10].
2. Iron oxide nanoparticles
IONPs stand out among various other NPs because of their low cost, superparamagnetic behaviour, biocompatibility and biodegradability [11, 12]. They have gained immense importance in the field of cancer therapy because of their superparamagnetic property, by virtue of which they can heat up when exposed to an external magnetic field or can be simply guided to a target site using an external magnet. IONPs are also the only metal oxide NPs approved for use in MRI [13].
Superparamagnetic iron oxide nanoparticles (SPIONPs) have various magnetic phases, of which magnetite (Fe3O4) and maghemite (γ-Fe2O3) are particularly promising in biomedicine [14, 15]. For example in MRI, slower renal clearance and higher relaxation values of SPIONPs compared to the conventionally used contrast agents like gadolinium-based contrast agents, make them more attractive for imaging purposes. Feridex, endorem, combidex and sinerem are some clinically approved SPIONPs for use in MRI [16].
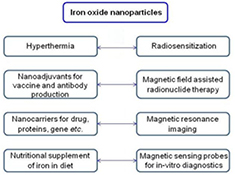
SPIONPs can also be easily targeted to tumor tissues using an external magnetic field. SPIONPs also find use in other biomedical applications like tissue specific release of therapeutic agents, enhancement of imaging and detection in MRI, hyperthermia based killing of cancerous cells, radiosensitization, magnetic field assisted radionuclide therapy, and in in vitro diagnostics as magnetic sensing probes [17–26]. They can be used for removing pollutants from ground water, nutritional supplementation of iron in diet as well for curing anemia, hemoglobin regulation and as nanoadjuvants for vaccine and antibody production, (figure 1) [27–29]. The best part about using SPIONPs in cancer therapy is that they get degraded and metabolized by the body and therefore do not accumulate in the body.
Figure 1. Applications of iron oxide nanoparticles in health and medicine.
Download figure:
Standard image High-resolution imageIONPs can be synthesized by various methods using chemical and biological processes. Co-precipitation method involves the use of iron salts which are precipitated as IONPs in aqueous basic solutions at room temperature or high temperature. However to obtain highly monodispersed IONPs, organometallic or coordinated iron precursors are thermally decomposed at high temperatures in organic solvents.
IONPs can be precipitated in nanodomains of water droplets dispersed in an oil phase by microemulsion method. They can also be synthesised electrochemically using electrolysis where iron anode is precipitated as IONPs in the electrolyte. Acoustic cavitation generated by ultrasound is also used for synthesis of IONPs. In laser pyrolysis, vapours of iron precursors are heated followed by controlled oxidation to obtain IONPs. Various biological organisms from microorganisms to plants have also been used for synthesis of IONPs. For details of the methods of synthesis of IONPs, see elsewhere [30, 31].
Bare IONPs thus synthesized have very small size and large surface energy, and are very reactive and unstable. In order to reduce their surface energy, these uncoated IONPs tend to aggregate. Therefore they are variously coated to prevent their aggregation. For example, IONPs coated with oleic acid, polymeric molecules like polyethylene glycol (PEG), polyvinyl alcohol (PVA) and inorganic materials like silica prevent/decrease aggregation, thereby increasing stability and biocompatibility [30]. Various other polymeric molecules such as polyvinyl pyrrolidine (PVP), polyacrylic acid, polystyrene, polymethyl methacrylate, polydipyrrole/dicarbazole, etc and natural dispersants like ethyl cellulose, dextran, citric acid, casein, chitosan, oleic acid, silica, starch, gelatin, etc have been used for coating IONPs to increase stability [32]. These coatings can also be conjugated with various ligands, proteins, drugs, enzymes, antibodies, fluorophores or nucleotides etc, for subsequent applications. For example, fluorophore-conjugated SPIONPs can be used for imaging or antibody-conjugated SPIONPs can be targeted to desired tissues or organs using an external magnetic field [33]. The coatings should however not affect the superparamagnetic behaviour of the SPIONPs, which is a desirable property of SPIONPs for tissue targeted therapy.
3. IONPs in breast cancer therapy
Despite various developments in the treatment and diagnosis of breast cancer, delay in diagnosis makes it still one of the most fatal cancers. Conventional treatment approaches include hormone therapy, immunotherapy, chemotherapy etc. Cancer immunotherapy involves the use of antibodies which bind to receptors and inactivate breast cancer cell growth signaling pathways. Commercial use of trastuzumab antibodies against human epidermal growth factor receptor 2 (Her2) is an example of successful use of cancer immunotherapy. In hormone therapy, breast cancer growth stimulating hormone, estrogen, is lowered in the body or estrogen receptors (ER) are blocked. For example, estrogen analog tamoxifen (TMX) is used to block estrogen from binding to ER and activate estrogen responsive genes that stimulate breast cancer cells growth. Both above mentioned therapies can however be used only in case of breast cancer cells expressing estrogen, progesterone or Her2 receptors. However breast cancer cells expressing none of these receptors i.e triple negative (TN) breast cancer cells are very aggressive and difficult to treat. Chemotherapy has been used to treat TN breast cancer, which has serious side effects due to non-specificity. However, due to the various limitations and side effects associated with these therapies, newer treatment strategies are being developed. NPs in breast cancer therapy are showing promising results [34, 35].
Among various NPs, IONPs with very unique properties are being evaluated to improve the treatment options available for breast cancer. Besides already being approved by The Food and Drug Administration (FDA) as contrast for MRI, they are being used in various ways ranging from nanocarriers for therapeutics, overcoming therapy resistance to killing cancer by hyperthermia.
3.1. Nanocarriers of therapeutics
3.1.1. Chemotherapeutic drugs.
IONPs have been extensively used as drug delivery systems (DDS) for chemotherapeutic drugs such as doxorubicin (DOX), TMX, quercetin, rhodium (II) citrate, gallic acid, etc. The drugs are loaded in such a manner that the functionality of the drug is not compromised. DDS are more effective in killing cancer cells compared to drugs alone due to the more effective targeting and entry of the IONP-drug complexes inside the cancer cells. This results in reduced drug doses and lesser side effects.
The advantages of using IONPs as DDS are that these complexes can be magnetically guided to desired tissues using external magnets or actively targeted by conjugating targeting ligands on their surface, or they can simply diffuse to tumor sites due to the enhanced retention and permeability (EPR) effect. The drug-IONPs are made such that on reaching the target tissues, they release the drugs in response to tumor microenvironment (TME) such as acidic pH or by magnetic field assisted drug release. IONPs have also been used for the targeted delivery of therapeutic payloads by conjugating them with ligands/antibodies specific for receptors/antigens, over expressed on the targeted cancer cells (discussed later).
Nanocomplexes formed by loading chemotherapeutic drugs such as DOX, TMX and quercetin onto IONPs were found to be efficiently internalized by MCF-7 cells (human breast cancer cell line) to show dose-dependent cytotoxicity [36–38]. Similarly, gallic acid loaded onto IONPs coated with PEG or PVA was found to be more toxic to breast cancer cell lines MCF-7 than free gallic acid, with no significant toxicity on normal cells [39]. Rhodium (II) citrate-IONP complexes were found to be more specific and cytotoxic to cancer cells (MCF-7 and 4T1 carcinoma breast cells) than normal cells (MCF-10A) and at doses lower than free rhodium (II) citrate. This may be due to a faster metabolism and hence a higher uptake of micronutrients such as iron in cancer cells. The carboxylic groups present in IONPs may also help in easy transport across the cell membrane through the proteic thiol groups [40].
Besides in vitro cytotoxicity, rhodium (II) citrate-IONP complexes caused significant reduction in tumor volume, higher tumor necrosis in Balb/c mice bearing orthotopic 4T1 breast carcinoma, compared to free rhodium (II) citrate or IONP-citrate complexes. These NP complexes were not toxic to the mice but due to effective delivery, accumulation and inhibition of glycolysis by citrate, they could effectively reduce tumor volume [41]. Intratumoral injection of L-ascorbic acid (vitamin C) coated magnetic nanoparticles (MNPs) in murine Ehrlich ascitis carcinoma (ESC) bearing mouse lead to effective accumulation in tumor tissues with significant reduction in tumor size, increase in necrotic areas than intraperitoneal administration. Both intratumoral and intraperitoneal injection lead to an enhanced expression of p53 and p16, which led to apoptosis of ESC cells. IONP complexes were also found helpful in overcoming drug resistance [42].
DOX loaded IONPs were more efficiently taken up by DOX-resistant breast cancer cells (1 µM dox resistant MCF-7) compared with free drug, thereby increasing the efficacy of the drug [43, 44]. Therefore, MNPs can increase the efficiency of chemotherapeutic drugs by reducing their dose due to direct accumulation in cancer cells and spare toxicity to the normal cells and tissues.
3.1.2. Small/short interfering RNA (siRNA).
IONPs carrying siRNA against genes that suppress the expression of the various functional and metabolic pathways of cancer cells have been developed and successfully used to inhibit the growth and progression of breast cancer. IONP-siRNA enters cell through cell transcytosis which is a safer and efficient way for siRNA delivery. IONPs carrying cyclooxygenase-2 (COX-2) siRNA have been used to downregulate COX-2 protein and the downstream signalling pathways in breast cancer, growth and progression in highly metastatic MDA-MB-231 breast cancer cells, and tumors respectively. These complexes further helped in optical and MRI detection of in vivo delivery [45]. Similarly, IONPs complexes with dual mode of siRNA delivery and imaging function have been used to image and decrease tumor growth in BT20 tumors (TN breast cancer cell line) using siRNA against anti-apoptotic gene BIRC5 [46], to reduce vascular endothelial growth factor (VEGF) both at the mRNA and protein levels, and also to enhance MRI in MCF-7 cells [47]. Further, to overcome multidrug resistance (MDR), SPIONPs carrying Pgp-siRNA (P-glycoprotein, a cell membrane protein involved in MDR) have been used to effectively knockdown P-glycoprotein (P-gp) and reduce the expression of P-gp, resulting in sensitivity of the breast cancer cells to adrinamicine [48].
3.1.3. Targeting ligands/antibodies.
IONPs have also been used for the targeted delivery of therapeutic payloads by conjugating them with ligands/antibodies specific for receptors/antigens, over-expressed on the targeted cells. These nanocarriers could be guided with the help of an external magnetic field to the target sites, thereby providing effective targeting to the desired tissues. This ensured more efficient uptake. Since there is more accumulation in the targeted cells and concentrations used are not toxic, the non-targeted cells are not affected, resulting in reduced side effects [49]. For example, variously coated IONPs have been tagged with herceptin for targeted delivery to human epidermal growth factor-2 receptor (Her2) and epidermal growth factor receptor (EGFR) overexpressing breast cancer cells. This helped in specific uptake of the nanocomplexes for drug delivery [50], hyperthermia [51, 52] or imaging and detection applications [53–57].
Folate tagged IONPs have also been used for targeted delivery to folate receptors overexpressing breast cancer cells for similar applications [58–60]. Targeted delivery effects only cancer cells expressing the targeted molecule, thereby decreasing the systemic toxicity and the side effects, some of which could be life threatening. AntiHer2 antibodies tagged IONPs have also been used as immunosensor to detect Her2 in serum samples as breast cancer biomarkers with excellent sensitivity [61]. Phosphatidylserine (PS) targeting monoclonal antibodies (MAb) have been tagged to SPIONPs to help target and bind to PS exposed tumor vessels to increase accumulation of SPIONPs in breast tumors and enhance tumor contrast. This increases sensitivity and specificity of detection of breast cancer at an early stage [62]. Endoglin/CD105 MAb tagged-IONPs loaded single wall carbon nanotubes (SWCNTs) allowed enhanced targeting to 4T1 breast tumors, increasing sensitivity of MRI detection and delivery of higher doses of thernostics nanocarrier SWCNTs [63]. IONPs tagged with EGFR-MAb have been used for dual imaging i.e. fluorescence molecular tomography (FMT) and MRI (both non-radiative and non-invasive) to enhance breast cancer detection. IONP-EGFR-MAb could bind efficiently to EGFR expressing human breast adenocarcinoma cell lines MDA-MB-231 and MDA-MB-231 tumors in mouse model to enhance MRI detection [64]. Iron oxide/Gold NP complex tagged with CD105 antibody were used to detect tumor angiogenesis in tumor-bearing mice by MRI [65]. Bombesin tagged IONPs could enhance MRI detection of T47D breast cancer cells due to specific binding of bombesin to T47D cells. In vivo studies performed using breast tumor mouse model showed higher accumulation of these nanocomplexes in tumors to significantly enhance MRI detection [66]. SPIONPs conjugated with VEGF could accumulate more efficiently in tumor tissues, thereby enhancing uptake and retention in tumor tissues compared to vascular endothelial growth factor receptor (VEGFR)-targeted SPIONP-liposomes and free SPIONPs. Therefore they could be used to enhance MRI detection of breast cancer tissues [67].
3.2. Imaging and detection
IONPs exhibit photochemical, photothermal and magnetic properties which makes them highly suitable for detection as well as diagnostic applications. Recently, use of SPIONPs as contrast agent to enhance MRI is getting popular and is already approved by FDA. Superparamagnetic nanoparticles such as maghemite (γ-Fe2O3) and magnetite (Fe3O4) nanocrystals are predominately used as T2 contrast agents. Advantages of using SPIONPs over conventionally used gadolinium complexes include higher contrast effects due to higher T2 relaxivity, higher blood retention time, biodegradability and low toxicity (toxicity of gadolinium based agents not known) [68]. Due to their superparamagnetic behaviour, they can also achieve higher magnetization values which results in enhanced proton relaxation and therefore less amount of SPIONPs are needed to provide dark contrast, high sensitivity, compared to gadolinium agents [69, 70]. SPIONPs have helped in accurately identifying metastatic nodes, image solid breast tumors as well as metastasis of breast cancer cells by combined positron emission tomography (PET)/MRI. Ultrasmall superparamagnetic IONPs (USPIONPs, Ferumoxtran-10/Combidex®,) have been used to accurately identify metastatic nodes in breast cancer patients by significantly enhancing the MRI of lymph node metastases [71]. IONPs have also been used to design long-circulating positron-emitting magnetic nanoconstructs (PEM) to image solid tumors for combined PET/MRI. These PEMs, consist of PEG coated phospholipid monolayer enclosing a poly lactic-co-glycolic acid (PLGA) core encapsulated USPIONPs. PEMs with their high transverse relaxivity enhance the imaging of breast cancer tissues [72].
SPIONPs have been used for MRI detection of metastases in sentinel lymph nodes (SLN) in breast cancer patients using computed tomography (CT) lymphography [73]. Gold standard for detection of SLN involves the use of a radioisotope (99mTc) alone or in combination with blue dye breast injection, using a gamma probe (GP). A new, non-radioactive method using SPIONs tracer (Sienna+®) along with a manual magnetometer (SentiMag®) has been developed for the detection of SLN. This method (Sienna+®) when compared with the radioisotope (99mTc) method for the detection of SLN in a multicentric and multinational non-inferiority study showed that both methods had equivalent efficiency for detection rates, however the new method was easy and safe [74–76]. Thus, detection of SLN using SPIONPs being non-radioactive is a safer and better method over using radiotracer 99mTc.
To detect metastasis of breast cancer cells, brain seeking metastatic human breast cancer cells, MDA-MB-231BRL or 231BRL, were labelled with ferumoxides-protamine sulphate (a complex of FDA approved SPION based imaging agent, ferumoxide and protamine sulphate). These magnetically labelled cells were then injected intracardiacally in female nude rats to develop metastatic breast tumors in the brain and were followed by MRI of the heads. Hypointense areas corresponding to SPIONP labelled 231BRL metastases and images with increased negative contrast (darker) in the brain were obtained compared to animals that received unlabeled cells. Prussian blue and immunohistochemical staining of brain sections further confirmed the presence of breast cancer cells in the brain sections indicating metastasis [77].
IONPs have also been used to identify new biomarkers for breast tumor initiating cells (BTICs). Extra domain-B of fibronectin (EDB-FN) is a tumor biomarker and has high expression in BTICs while non-BTICs show no expression of EDB-FN. SPIONs conjugated with EDB-FN specific ligand could effectively target and bind selectively to the BTICS to enhance MRI and identify BTICs at the same time [78].
To understand metastatic process magnetic MNPs have been used as imaging probes for the real-time monitoring of the epithelial-mesenchymal transition (EMT) in cells. During EMT, the mesenchymal phenotype is indicated by the increased expression of e-box-binding homeobox 1 (ZEB1) mRNA. The increase in ZEB1 mRNA and thus EMT is shown by using a molecular beacon (MB), miR-200a-MBMNPs (MNPs tagged with miR-200a) which can bind to ZEB1 mRNA and fluoresce. Mammary epithelial cells (NMuMG) treated with TGF-β1 to induce EMT (increase ZEB1 expression) were incubated with the MB and a restoration of fluorescence of the internally quenched MB fluorophore was seen, resulting in an increase in the fluorescence intensity and thus better monitoring of EMT [79].
A nanocomplex consisting of IONPs coated with dimercaptosuccinic acid (DMSA) and conjugated with glucose analog 2-deoxy-D-glucose (2-DG) (γ-Fe2O3@DMSA-DG) was used for better detection of tumors with high glucose metabolism in MRI. These NP complexes were most efficiently taken up by high glucose metabolizing breast cancer cells MDA-MB-231 due to the conjugated 2-deoxy-D-glucose (2-DG) ligand which binds to the glucose transporter, with no toxicity. Further nude mice bearing human MDA-MB-231 breast cancer xenografts when injected with γ-Fe2O3@DMSA-DG NPs also showed significant uptake and increased sensitivity to detection [80]. IONPs have also been used as biosensors for detection of breast cancer cells in whole blood, at frequencies around 20 kHz by measuring relative impedance changes. A bioconjugate comprising of ferromagnetic nanoparticles (IONPs) labelled with fluorescein isothiocyanate (FITC), and coated with a layer of silica coupled with mouse MAb c-erbB-ab15 was found to efficiently bind to c-erbB-2 proteins characteristically expressed on BT-474 breast cancer cells. These bioconjugate-bound tumor cells were separated using a magnet to form an interphase of tumor cells in the proximity of gold electrodes with a complete electrical circuit. When a signal at a range of frequencies was passed through the circuit, an impedance changes around 20 kHz were observed. Thus, this technique could be used to measure relative impedance changes to detect circulating tumor cells (breast cancer cells) in whole blood, at frequencies around 20 kHz [81].
3.3. Thermotherapy (hyperthermia)
Hyperthermia is one of the many treatment modalities used in cancer therapy. It is used to kill cells by exposing them to elevated temperatures ranging from 41 °C to 46 °C [82]. SPIONPs injected into the tumor tissues can be heated up using an external magnetic field. In the presence of the magnetic field, the SPIONPs start to vibrate and generate heat, which in turn destroys the tumor cells. IONPs have been used to kill cancer cells by hyperthermia alone or in combination with drugs which is discussed in combination therapies later. Table 1 compiles the literatures on use of IONPs in killing breast cancer cells by heating them up to hyperthermic temperatures.
Table 1. IONPs in hyperthermia mediated cancer therapy.
| Nanoparticles | Cancer cell lines/tumor model used | Observed effects | Reference |
|---|---|---|---|
| Starch coated MNPs | CRL-1666 cells and CRL-1666 tumor xenografts in female Fischer rats | Effective ablation of metastatic tumors in spine | [83] |
| Carboxymethyl dextran coated IONPs (IO-CMDX) | MCF-7 cells | Magnetic fluid hyperthermia using IO-CMDX was more effective in killing the cancer cells than hot water hyperthermia | [84] |
| Superparamagnetic and ferromagnetic magnetite NPs | MCF-7 cells | Greater heating and cell killing capacity of ferromagnetic NPs compared to superparamagnetic NPs with same uptake | [85] |
| Uncoated IONPs (bionized nanoferrite nanoparticles MicroMod GmBH Rostock, Germany) | Female C3H mice bearing MTGB tumors | MNP induced hyperthermia (mNPH) caused slower tumor regrowth in mice with reduced normal tissue damage compared to 915 MHz microwave hyperthermia at the same thermal dose | [86] |
| Dextran-coated MNPs | Murine breast adenocarcinoma (MTG-B) tumor bearing C3H/He mice | Combination treatment of hyperthermia and radiation more effective in tumor growth suppression. MNP hyperthermia more effective than microwave mediated hyperthermia | [87] |
3.4. Combination therapy
IONPs in addition to drugs can also carry a variety of molecules to act as multifunctional nanocarriers. IONPs have been used to kill cancer cells using a combinatorial approach of hyperthermia and chemotherapy (drugs such as cinnamaldehyde, selol, DOX, alendronate, docetaxel, etc). The advantage of using combination is that higher toxicity is observed at doses lower than in these therapies alone, thus minimizing side effects.
Cinnamaldehyde tagged IONPs reduced the viability of breast cancer cell lines (MCF-7 and MDA-MB-231) at doses lower than free cinnamaldehyde and could heat up to hyperthermic temperatures. Cinnamaldehyde-IONPs could therefore be used to enhance killing of cancer cells through a combined approach of hyperthermia and a lower therapeutic dose of drug, resulting in reduced toxicity to normal cells [88]. Similarly, Selol-IONP nanocomplexes were found to be more cytotoxic than free selol to neoplastic breast cell lines (4T1 and MCF-7) [89].
Nanocomplexes such as DOX-IONP and alendronate-SPIONPs were also more effective in killing MDA-MB-231 breast cancer cells using a combinatorial approach of chemotherapy and hyperthermia than hyperthermia or drug alone [90]. These nanocomplexes were able to reduce the tumor growth in nude mice bearing MDA-MB-231 tumors compared to free drug. They had significant superparamagnetic properties to be used in MRI [91, 92].
Compared to a single or dual modality of therapy, herceptin tagged drug-IONP complexes were found to be more effective in killing the cancer cells since it combines chemotherapy, hyperthermia and specific targeting to cancer cells to show synergistic effects. Nanocomplexes of docetaxel, IONPs and herceptin were found to show higher uptake by HER2-positive SK-BR-3 breast cancer cells compared to complexes without herceptin [93]. SPIONPs loaded with TMX and tagged with folic acid (FA) could heat up to hyperthermic temperatures when exposed to an alternating magnetic field (AMF) which also lead to TMX release. Thus these nanocomplexes could be used for hyperthermic killing of cancer cells combined with chemotherapy and specific targeting [94]. To suppress the expression of MDR-associated proteins (MRP 1 and 3), IONPs were utilized in a combinatorial approach. When BT-474 adenocarcinoma cells were sequentially exposed to MNPs, hyperthermia and mitomycin C, there was reduction of expression of membrane MRP. This is a very useful approach to develop strategies to overcome MDR [95].
A number of chemotherapeutic drugs such as cisplatin, DOX, β-cyclodextrin, curcumin etc, have been conjugated with IONPs to develop multifunctional NPs that can be used for both imaging (diagnosis) and drug delivery (therapy). In contrast to IONPs that deliver only a single payload of drug or active agent, multifunctional NPs can integrate various functionalities to synergistically achieve maximal anti-tumoral activity. NP-drug complexes have shown to significantly enhance MRI detection, show sustained drug release and kill cancer cells at much lower doses than when drugs are used alone [96, 97].
β-cyclodextrin, IONP and anti-cancer drug curcumin (Cur) nanoformulation has been used to mediate hyperthermia, MRI and drug delivery. This nanoformulation showed superior heating ability and imaging contrast properties with enhanced uptake and inhibitory effect similar to curcumin on MCF-7 breast cancer cells compared to bare uncoated IONPs and β-cyclodextrin coated IONPs (CD200) [98].
IONPs have also been used for chemo-phototherapy. IONPs carrying a drug and a photodynamic agent to mediate chemotherapy and photodynamic therapy (combination therapy) showed higher therapeutic effects using rather low doses of therapeutic agents [99]. Similarly, IONPs decorated with gold NPs (can absorb near infrared radiation) and loaded with DOX when irradiated with near infrared irradiation showed an IC50 value lower than free DOX or DOX-loaded NP complexes without irradiation. These nanocomplexes could also enhance the MRI of tumors significantly and were therefore effective as carriers of drug, photothermal agents and contrast agents in imaging [100].
IONPs conjugated to radiopharmaceutical agent 177lutetium-trastuzumab, have been used for the dual purpose of radioimmunotherapy (RIT) for treatment, and estimation of dose of therapeutics delivered to tumors and critical organs using MRI. 177lutetium-trastuzumab IONPs or 177Lu-trastuzumab-NPs injected into breast tumor bearing BALB/c mice could effectively aggregate in the liver with no specific accumulation in other organs. Due to high sensitivity of the IONP complex in MRI, liver and tumor activity estimation using MRI images were also close to real amounts of therapeutics given [101].
3.5. Enhancement of radiation therapy
Radiation is one of the major treatment approaches in cancer therapy. However, there are number of challenges associated with this treatment regime, like possible injury to surrounding tissues, differential exposure of tumor depending on accessibility, and development of radioresistance [102]. Since acquired radiation resistance is one of the major reasons of failure of radiotherapy, strategies focusing on reversal of radiation resistance or radiosensitization can be useful. This can be done by targeting signalling pathways or specific proteins associated with radiation resistance. Another hurdle in radiation therapy is the presence of hypoxic core which renders the portion of tumors/cells resistant to therapy. To overcome these limitations, IONPs have come up as an important therapeutic tool to enhance breast cancer radiation therapy.
Murine breast cancer cells (MTG-B cells) incubated with starch coated IONPs alone did not show any cytotoxicity. However γ radiation of these breast cancer cells resulted in post irradiation cytotoxicity. This may be due to scattering of the radiation by the metal atoms of IONPs which results in more effective targeting and damaging of cellular components [103]. Besides the combined effect of hyperthermia and radiation therapy on breast cancer in vivo has been studied by injecting IONPs into MTG-B tumors in female C3H mice and exposing them to radiation and then to an AMF to generate heat. Tumor growth was found to get delayed to a great extent when hyperthermia and radiation was given together compared to radiation or hyperthermia alone [104]. The radiosensitizing potential of IONPs was further studied by incubating citrate coated and uncoated IONPs with MCF-7. Both IONPs were efficiently internalized by the cancer cells and the cell viability decreased only slightly. Exposure of these IONPs containing MCF-7 cells to x-ray radiation resulted in enhanced reactive oxygen species (ROS) production. Thus, IONPs can be used for radiosensitizing cancer cells via enhanced ROS production [22]. Use of NPs like IONPs for reversal of radioresistance in cancer cells is an area in cancer therapy which needs to be explored. Although IONPs have been reported to act as radiosensitizers in cancer cells, their ability to overcome radioresistance has not been studied. IONPs are reported to oxygenate hypoxic tumor under hyperthermic conditions, however their effect on tumor hypoxia (without hyperthermia), has not been studied. Therefore, the modulatory effect of IONPs on radioresistant cancer cells and role under hypoxic conditions needs to be evaluated. Using metal NPs like IONPs in radiation therapy also gives the added advantage that metal NPs selectively scatter and/or absorb the high energy x-ray or γ-ray radiations. This allows for better targeting of cellular components within the tumor tissues, resulting in more localized and consolidated damage.
4. Mechanism of action of IONPs
4.1. Iron overload and induction of reactive oxygen species (ROS)
Magnetically targeted IONPs accumulate in the targeted tissues which can lead to iron overload and thus imbalance in iron haemostasis. The excess iron ions lead to overproduction of ROS which is toxic to the cells.
IONPs once internalized by the cells enter the lysosomes where they are broken down in the acidic environment into free iron ions. These iron ions are then incorporated in the body in the form of proteins like ferritin, haemoseridin, etc [105]. Excess free iron ions generate ROS by participating in The Haber–Weiss reaction and Fenton reaction [106]. The increased ROS production induces oxidative stress. This results in DNA damage, oxidative stress, epigenetic events, inflammatory processes and cytotoxicity [13]. Free iron ions through Fenton reaction have been reported to cause promotion of cancer from stage-I to stage-II in murine skin [107]. IONPs have been found to decrease cell viability, increased lactate dehydrogenase leakage, ROS and lactoperoxidase levels and depleted glutathione, superoxide dismutase, and catalase in a concentration and time-dependent manner in human breast cancer cells (MCF-7) [108]. Induction of ROS by IONPs and cell death has also been reported in several other studies [109–111].
4.2. Hyperthermia
IONPs can kill the cancer cells by hyperthermia also. IONPs because of the superparamagnetic properties can be magnetically targeted to the tumor sites or directly injected into the tumor tissues. These accumulated IONPs when exposed to an oscillating magnetic field, absorb the energy, start to vibrate and then convert them into heat energy. The heat generated by the IONPs is significantly high (above 42 °C) and therefore destroys the tumor cells [112]. The advantage of using IONPs in hyperthermia is that the heat is generated internally and only in the desired tissues. Also cancer cells are more sensitive than normal cells to hyperthermic temperatures [113]. The use of IONPs to kill cancer cells by hyperthermia have been reported [83–87].
4.3. Mechanical movement
IONPs exposed to oscillating magnetic field can also kill cancer cells in non heat mediated mechanism. IONPs labelled breast cancer MDA-MB-231 cells when exposed to oscillating gradients of strong magnetic field showed damaging effects. The destruction of cells was due to the aggregation and mechanical movement of IONPs [114].
5. Conclusion and future perspectives
The impact that NPs can have in cancer nanotherapy is yet to be fully explored. NPs, particularly IONPs, have great potential to enhance treatment of breast cancer. They increase the sensitivity of breast cancer cells to MRI detection, hyperthermia, chemotherapy, radiotherapy and photodynamic therapy. The unique property of IONPs to act as diagnostic and a therapeutic agent simultaneously needs to be exploited further to develop low cost drugs. IONPs can be developed as an important thernostic tool for early detection which is the mainstay of prevention and better patient survival in breast cancer. Therefore more of thernostics multifunctional IONPs with very high saturation magnetization and ability to incorporate biological ligands need to be developed in the future.
The use of IONPs in reversal of radioresistance in cancer cells is an area in radiotherapy which needs to be explored. Successful clinical translation of these IONPs formulations is the most important challenge to overcome in nanotherapy. Further, more studies on the toxicity of IONPs and their mechanisms need to be done.
Therefore, it can be concluded that IONPs possess the versatility required to overcome some of the most challenging impediments in treatment of cancers as a whole and breast cancer in particular.
Acknowledgments
Grants received from Jawaharlal Nehru University, India in the form of UGC-Networking, UPE-II, DST-PURSE is gratefully acknowledged. ST is thankful to UGC for providing financial support.