Abstract
Over the past decades, biopolymer-based nanomaterials have been developed to overcome the limitations of other macro- and micro- synthetic materials as well as the ever increasing demand for the new materials in nanotechnology, biotechnology, biomedicine and others. Owning to their high stability, biodegradability, low toxicity, and biocompatibility, biopolymer-based nanomaterials hold great promise for various biomedical applications. The pursuit of this review is to briefly describe our recent studies regarding biocompatible biopolymer-based nanomaterials, particularly in the form of dendrimers, hydrogels, and hydrogel composites along with the synthetic and modification approaches for the utilization in drug delivery, tissue engineering, and biomedical implants. Moreover, in vitro and in vivo studies for the toxicity evaluation are also discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since their emergence biopolymer-based nanomaterials have been attracting much attention due to their novel characteristics such as excellent electrical, magnetic, and catalytic properties; increased strength and chemical reactivity compared to the traditional macro- and micro-materials [1–12]. These capabilities provide ideal materials for various applications including aerospace industry, telecommunication industry, electronics, agriculture, and especially for biomedical applications such as drug delivery system, anticancer drugs/prodrugs and tissue engineering [2, 8, 11–18]. However, newly synthetic nanomaterials may also pose toxicological risks to healthy cells due to their low biocompatibility as regards the applications in biomedical field [19, 20]. As a result, a number of fascinating strategies have been developed in design, synthesis and modification of nanomaterials to enhance their biocompatibility.
In recent years, the development of renewable and biodegradable polymer materials becomes a significant area of biomedical research [3, 21]. Owing to its stability, low toxicity, biodegradability, renewable nature and biocompatibility properties, biopolymers attract many investigations [21–23]. In fact, nanomaterials that are synthesized or modified by biopolymers, are proven their potential to deliver drugs in targeted, controlled and sustained manner, promising for pharmaceutical drug delivery [24, 25]. Besides, many great specific characteristics, that are necessary for the synthesis of hydrogels/nanocomposite hydrogels comprise highly porous structure, conformable mechanical properties and tissue regeneration, are also expressed in biopolymers [26, 27]. Furthermore, these biopolymer-based nanocomposites have the mass degradation rate that is within the range needed for bone regeneration [28, 29].
This topical review summarizes results of scientific research in Pharmaceutical Chemistry Department, Institute of Applied Materials Science, Vietnam Academy of Science and Technology. Herein, we focus on biocompatible polymer-based nanomaterials, particularly dendrimers, hydrogels and nanocomposite hydrogels as well as different methods for synthesis, modification, targeted drug delivery and other biomedical applications In addition, the formation of dendrimer-platin/platinum-based drugs complexes, tissue and bone regeneration along with characterization techniques and their toxicity evaluation are presented.
2. Biocompatible nanomaterials based dendrimers
The family of polymer-based nanoparticles and nanostructured materials consists of polymeric nanomicelles, polymersomes, nanocapsules, nanospheres, and dendrimers. Recently, we have paid our attention to dendrimers by virtue of their unique physicochemical properties for a wide variety of applications [27, 30]. Dendrimers are the polymeric architectures that belong to the family of hyperbranched polymers. These were explored by Tomalia in 1980's [31] and have known for their molecular well-defined structure, high functionality, and versatility [30–32]. Dendrimers have been suggested as a novel and 'smart' nanocarrier because of the internal cavities inside their structure, which facilitate entrapping and conjugating of both hydrophobic and hydrophilic biomolecules [32, 33]. Owing to these characteristics, dendrimers play an important role in the development of new drug delivery systems. However, the major limitation of dendrimers for their use in the biological system is the accompanied toxicity [32, 34]. Considering this difficulty, our review will find out different solutions to mitigate the inherent toxicity along with associated characterization methods for the toxicity evaluation.
2.1. Synthesis, modification and applications of dendrimers for drug delivery and targeting
There are various types of dendrimers, depending on the synthetic strategies and modification methods to synthesize the dendrimer designs. Some main types of dendritic platforms have been produced and used as targeted drug delivery systems such as polyamidoamine (PAMAM), poly(propylene imine)-PPI, poly L-lysine, polyester etc [32]. Among them, PAMAM is one of the most attractive and common dendritic polymers [35–37]. PAMAM is a dendritic polymer that has attracted much attention in biomedical applications related to its high drug loading efficiency and externally exposed carboxylic or amino groups for the further modification with drugs, targeting agents or alkyl agents. PAMAM has proven its ability to enhance the solubility, stability and antitumor activity of chemotherapy drugs such as 5-fluorouracil, paclitaxel, 6-mercaptopurin, camptothecin, and methotrexate [34]. However, the use of this dendrimer is often restricted because of the strong interaction between the positively charged amino groups within the PAMAM and the negatively charged groups in the cell membrane that leads to cell lysis and hemolytic toxicity [34, 38]. Therefore, modifying PAMAM is necessary for reducing the cellular cytotoxicity and increasing the biocompatibility with the capability of delivering drugs.
2.1.1. Increasing biocompatibility of dendrimers by modification with alkyl agents
Alkyl agents such as ROH, RCOOH, RCOCl, RNH2 (R = ethyl, hexyl, octyl, dodecanoyl) were used to alkylate the surface of PAMAM. The alkylation may lead to an increase of the biocompatibility of the dendrimers, thus reducing the harmful effects to healthy cells. The obtained results obviously showed that alkyl-PAMAM can significantly reduce the cytotoxicity as compared with non-modified PAMAM, and furthermore indicated that PAMAM with longer alkyl chains can reduce toxicity more effectively than that with shorter alkyl chains.
2.1.2. Surface modification of PAMAM by polyethylene glycol (PEG) and pluronic to enhance the biocompatibility and efficacy for drug delivery
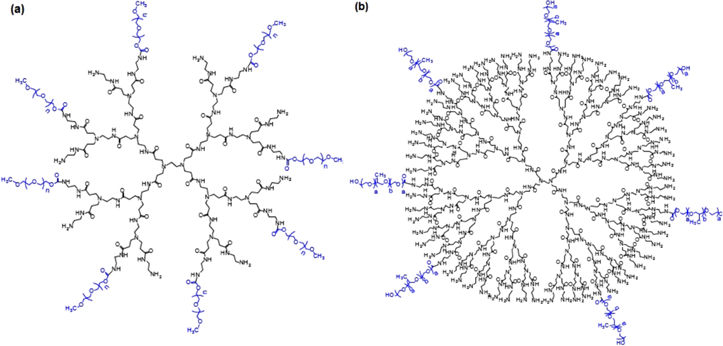
The modification of PAMAM by using four grades of polyethylene glycol (PEG 4000, PEG 6000, PEG 10000 and PEG 12000) and pluronic with different molecular weights (P123, F68, F127 and F108) were performed via the reactions between the amino groups of PAMAM and the hydroxyl groups of NPC-activated PEG or NPC-activated pluronic. Obtained products including PAMAM-PEG or PAMAM-pluronic are shown in figure 1. All PAMAM-PEG expressed higher biocompatibility than PAMAM, regardless of different grades of PEG. Moreover, the results also evidently showed that PAMAM-PEG with longer chains of PEG exhibited higher encapsulation degree of 5-fluorouracil (5-FU) than those with short chains of PEG [36].
Figure 1. Molecular structures of (a) PAMAM–PEG and (b) PAMAM-pluronic.
Download figure:
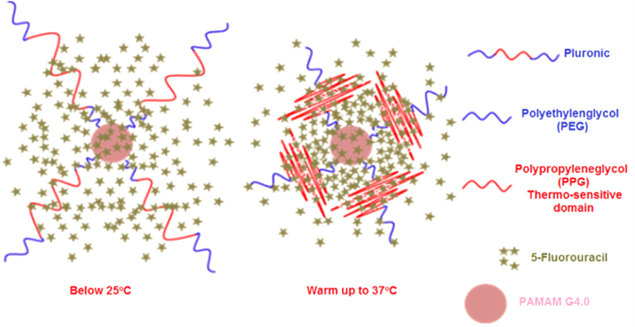
Standard image High-resolution imageThe loading efficiency of PAMAM.G4.0 5-FU (w/w) was only 9% while it was approximately 15% of 5-FU (w/w) in PAMAM.G4-PEG4000. In vitro, 5-FU was slowly released from PAMAM/5-FU and reached about 62% after the first 2 h, 82% after 5 h, resulting in the prolongation of the therapeutic drug's biological effects. In addition, as pluronic are temperature-responsive polymers, PAMAM-pluronic can increase its stability and slow down the release of 5-FU in physiological condition (figure 2). MTT assay showed the reduced cytotoxicity effect of PAMAM as compared with PAMAM.G4-pluronic F127, from 35.49 ± 3.93% to 4.20 ± 4.15%, as shown in table 1. Our results also demonstrated that the cytotoxicity of PAMAM.G4-F127/5-FU, similar to PAMAM.G4-PEG/5-FU, can reduce the cytotoxicity of 5-FU by 10 times.
Figure 2. Schematic presentation of drug-loaded PAMAM-Pluronic F127 at temperature below 25 °C and above 37 °C.
Download figure:
Standard image High-resolution imageTable 1. Antiproliferative activity (%) of PAMAM in MFC7 cells.
| Sample | Concentration (μg ml−1) | Antiproliferative activity (%) |
|---|---|---|
| PAMAM G4 | 100 | 35.49 ± 3.93 |
| G4-PEG6K | 100 | −2.92 ± 2.05 |
| G4-F127 | 100 | 4.2 ± 4.15 |
| 5FU | 1000 | 50.0 ± 1.6 |
| 5FU + G4-PEG6K | 3330 | 63.00 ± 1.72 |
| 5FU + G4-F127 | 800 | 65.36 ± 0.67 |
| 5FU + G4-PEG4K | 2200 | 91.01 ± 1.09 |
Additionally, PAMAM/5-FU can decrease the tumor volume by 30% after 5 days of incubation with MFC7 breast cancer cells. Meanwhile, there was no significant change in tumor volume in case of control sample and free drug. After 10 days of incubation, the tumor volume reduction was 16.62% for the free 5-FU while that was 44.90% for PAMAM/5-FU. Finally, approximately 42.30% of the tumor volume was reduced by the free 5-FU, whereas it was 74.67% for the PAMAM/5-FU after 15 days (figure 3).
Figure 3. In vitro cytotoxicity profiles of PAMAM-PEG/5-FU in MCF7 breast cancer cell line.
Download figure:
Standard image High-resolution image2.1.3. PAMAM synthesis for the active targeted delivery of anticancer agents
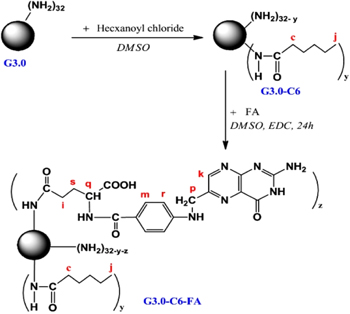
PAMAM-PEG-folic acid (FA)/5-FU, PAMAM-pluronic-FA/5-FU and PAMAM-hexyl-FA/5-FU were successfully formulated with the introduction of FA as for the targeting efficiency improvement. Figure 4 shows the synthetic diagram of PAMAM-hexyl-FA.
Figure 4. Synthetic scheme of PAMAM G3.0-hexanoyl-folate.
Download figure:
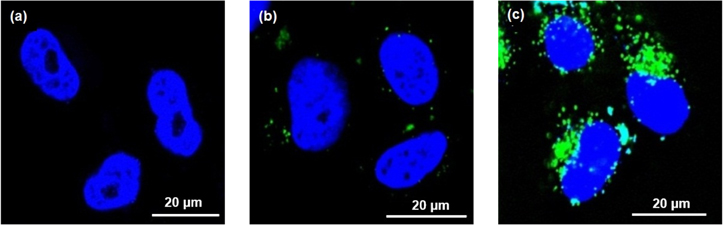
Standard image High-resolution imageThe systems were labeled with fluorescein isothiocyanate (FITC) to examine the cellular uptake of PAMAM G3.0-C6-FA dendrimer derivative. Analytic results of confocal microscopic images showed that the tumor uptake level of the PAMAM G3.0-C6-FA-FITC/5-FU (conjugated with FA) was higher than that of the PAMAM G3.0-C6-FITC (not conjugated with FA) (figure 5(b)). Primarily, this result confirmed that the FA targeting ligand was successfully conjugated to the PAMAM system that affects the cellular uptake and increases the antitumor efficacy of PAMAM G3.0-C6-FA. This can be explained by the presence of FA on the surface of dendrimer system that directs them to the targeted cancer cells where FA receptors normally overexpressed (figure 5(c)).
Figure 5. Confocal microscopic images: (a) control HeLa cells, (b) HeLa cells incubated with PAMAM G3.0-C6-FITC and (c) HeLa cells incubated with PAMAM G3.0-C6-FA-FITC.
Download figure:
Standard image High-resolution image2.2. Synthesis of PAMAM dendrimer-cisplatin complex as anticancer drugs and prodrugs
Platinum (Pt)-based anticancer drugs have been used for a long time, among them cisplatin is a widely prescribed one. It had a potential clinical impact, especially on human ovarian and lung cancer treatments, but the effectiveness of cisplatin is restricted by many side effects and drug resistance. Therefore, a dendrimer-based nanocarrier for loading and releasing cisplatin is developed rather than Pt-based drugs. By applying this nanocarrier system in vivo, cisplatin can be remained in circulation longer and be accumulated in targeted tumor via enhanced permeability and retention (EPR) effect [39, 40].
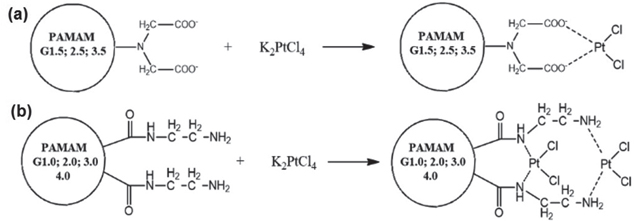
On the other hand, there are many types of anticancer drugs such as cisplatin, carboplatin or oxaliplatin that can be chemically conjugated with PAMAM dendrimer via its peripheral end-groups, leading to the formation of prodrugs. Herein, dendrimer-cisplatin complexes were synthesized by using PAMAM dendrimers and activated derivatives of cisplatin, in which PAMAM with terminal or surface amino groups and carboxyl groups that can form a Pt complex (figure 6).
Figure 6. Complex formation between K2PtCl4 and (a) half-generation PAMAM with ester groups on the surface, and (b) full-generation PAMAM with amino groups on the surface.
Download figure:
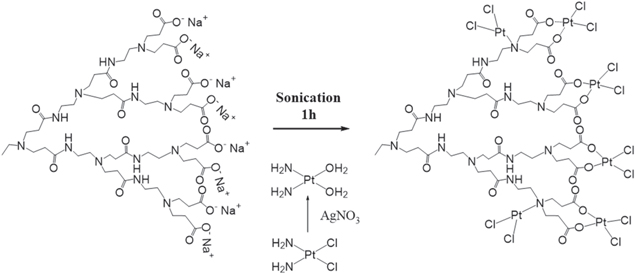
Standard image High-resolution imageThe synthetic reaction of the prodrugs from half-generation PAMAM and cisplatin is presented in figure 7, in which cisplatin was activated by silver nitrate (AgNO3) and functioned as a cross-linker. In addition, sonication was used in the synthetic procedure to increase the load of cisplatin [34].
Figure 7. Formation of carboxylated PAMAM G3.5-activated cisplatin complex [34].
Download figure:
Standard image High-resolution imageFurthermore, it has been proven that the PAMAM dendrimer-cisplatin complex performed highly effectiveness in cytotoxicity studies with NCI-H460 lung cancer cells. The obtained cytotoxicity results of PAMAM, cisplatin, and PAMAM-cisplatin complex at equivalent concentration of 100 μg ml−1 (table 2) demonstrated that the cytotoxicity degree of half-generation PAMAM with the surface carboxyl groups (e.g. PAMAM G3.5) was about −7.73 ± 0.20%. This revealed that half-generation PAMAM was completely non-toxic to NCI-H460 cells. In contrast, full-generation PAMAM with amino groups on the surface (e.g. PAMAM G3.0) showed a little toxicity effect at the screening concentration (4.99 ± 5.04%). As shown in table 2, K2PtCl4 was toxic to NCI-H460 cells (49.31 ± 1.57%), but the cells growth inhibition capability was not sufficient (less than 70%). When K2PtCl4 formed a complex with PAMAM 3.0 to fabricate PAMAM G3.0-Pt2+, meanwhile, inhibited 77.23 ± 0.73% cell growth and can be used as an anticancer drug. Moreover, the other complexes of PAMAM dendrimer-cisplatin including PAMAM G3.0-cisplatin, PAMAM inactivated cisplatin, and PAMAM G3.5-acitvated cisplatin also effectively inhibit 77.23 ± 0.73%, 72.15 ± 0.58%, and 96.21 ± 0.62% cell growth, respectively. These results implied the potential of PAMAM dendrimer-cisplatin complexes as anticancer prodrugs for the delivery of platinum-based anticancer agents.
Table 2. Cytotoxicity profiles of PAMAM, free cisplatin and PAMAM-cisplatin complex at equivalent concentration of 100 μg ml−1 against human lung carcinoma cell line NCI-H460.
| Antiproliferative activity (%) | ||||
|---|---|---|---|---|
| Sample | Group 1 | Group 2 | Group 3 | Mean ± STD |
| PAMAM G2.0 | 4.67 | −7.52 | −1.50 | −1.45 ± 6.09 |
| PAMAM G3.0 | 10.59 | 0.82 | 3.55 | 4.99 ± 5.04 |
| PAMAM G2.0/Pt | 80.18 | 77.19 | 77.20 | 78.19 ± 1.73 |
| PAMAM G3.0/Pt | 77.90 | 77.34 | 77.23 | 77.23 ± 0.73 |
| PAMAM G3.5 | −7.73 ± 0.20 | |||
| K2PtCl4 | 49.38 | 47.71 | 50.84 | 49.31 ± 1.57 |
| Cisplatin | 100 | |||
| PAMAM G3.0-Pt2+ | 77.23 ± 0.73 | |||
| PAMAM G3.5-cis-Pt (inactivated cisplatin) | 72.15 ± 0.58 | |||
| PAMAM G3.5-cis-Pt (activated cisplatin) | 96.21 ± 0.62 | |||
3. Synthesis and applications of hydrogels in biomedicine
Hydrogels are cross-linked polymer networks with the hydrophilic structure allowing them to absorb large amounts of water [7, 41]. Recently, biopolymer-based hydrogels and hydrogel composites with an excellent biocompatibility and biodegradation has proven to be promising potential for the applications in minimally invasive surgical implantation, dermal wound treatment, formation of a biomimetic microenvironment for catilage and bone tissue regeneration, and also drug delivery system [11, 26, 28, 42, 43].
3.1. Synthesis of dendrimer-tyramine-tetronic-based hydrogel and its application in targeted drug delivery
Cationic PAMAm dendrimers have several advantages for drug delivery such as high drug loading efficiency or highly electrostatic interaction with anionic bioactive agent, enhancement of hydrophobic drugs solubility, sustained drug release, and simple functionalization of their external groups to develop drug-loaded biomaterials. Therefore, versatile dendrimers platforms offer valuable tools for preparing many cationic hydrogels which would be highly significant in drugs delivery and tissue engineering. In this report, potential PAMAM-HPA-TTe-based hydrogels to carry heparin-anionic drug for coating stent or blood contacting devices were achieved.
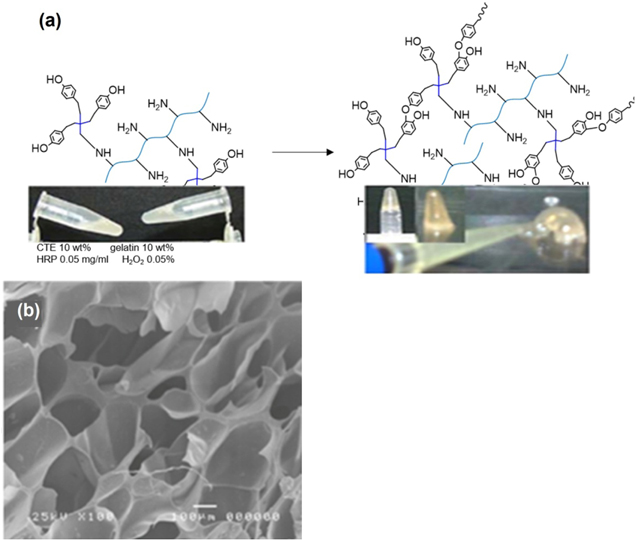
Tyramine-tetronic (TTe) copolymers were firstly synthesized while the cationic PAMAM dendrimers were grafted with p-hydroxyphenyl acetic acid (HPA) to form PAMAM dendrimers-HPA (Den-HPA). Then in the presence of enzyme horseradish peroxidase enzyme (HRP) and hydrogen peroxide (H2O2), the aqueous TTe and Den-HPA copolymer solution formed the cationic dendrimer-based hydrogels, as shown in figure 8. Characterization of heparin-encapsulated hydrogels demonstrated a high stability as well as the sustainable release of heparin, indicating that these hydrogels are the promising candidates for the controlled delivery of anionic drugs [11].
Figure 8. Synthetic scheme of heparin-loaded PAMAM-HPA-TTe hydrogels [11].
Download figure:
Standard image High-resolution image3.2. Synthesis of biocompatible polymer-based hydrogels (chitosan and tyramine-tetronic) and its application for dermal wound healing
Chitosan and tyramine-tetronic (CTTe) based hydrogels were prepared by HRP/H2O2-mediated coupling reaction as described in figure 9(a).
Figure 9. (a) Schematic formation of CTTe hydrogels after one minute and (b) SEM images of the lyophilized CTTe hydrogels [7].
Download figure:
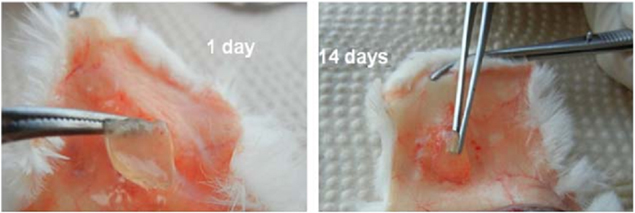
Standard image High-resolution imageThe lyophilized CTTe hydrogels exhibited uniformed structure with the average pore size in range of 200–300 μm (figure 9(b)). These hydrogels were appropriate for the delivery of drug and wound healing. Evaluation of biocompatibility of CTTe hydrogels was performed in vivo on Sprague-Dawley (SD) rats. The results showed that there was no abnormal phenomenon around the skin incision after one day of treatment and then the microvasculatures were observed on the skin incision covered with formed CTTe hydrogels after 14 days (figure 10). These obtained results expressed high potential of CTTe hydrogels for the skin wound healing [7].
Figure 10. Representative images of skin incision in SD rat after covered 1 day (left) and 14 days (right) with in vivo formed CTTe hydrogels [7].
Download figure:
Standard image High-resolution image3.3. Synthesis of biocompatible polymeric derivatives based hydrogels (chitosan oxidized and PEG-tyramine) as tissue adhesive material
The biocompatible polymer-based hydrogels (oxidized chitosan-based hydrogels) were fabricated via the grafting of activated polyethylene glycol-tyramine (PEG-TA) copolymer onto the oxidized chitosan (Ox.Ch) backbone under the presence of enzyme horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) as catalysts. In this synthetic process, chitosan was oxidized to improve the tissue adhesion characteristic of the oxidized chitosan–PEG-TA (Ox.Ch-PEG-TA) hydrogels.
Tissue adhesion and wound healing effects of Ox.Ch-PEG-TA hydrogels were examined on SD rats at University of Medicine and Pharmacy, Ho Chi Minh City. After 3 days of surgery, it is revealed that while the cyanoacrylate adhesive-treated wound was dry and left 'open' (figure 11(a)), the skin edges of the Ox.Ch-PEG-TA hydrogels-treated wound were sutured together without any inflammation (figure 11(b)), similar to the surgical suture-treated wound (figure 11(c)). Therefore, Ox.Ch-PEG-TA hydrogels can potentially be utilized for the treatment of dermal wounds instead of using surgical sutures [43].
Figure 11. Wounds were sealed with (a) cyanoacrylate adhesive, (b) Ox.Ch-PEG-TA and (c) surgical suture 3 days after closure [43].
Download figure:
Standard image High-resolution image4. Synthesis of nanocomposites hydrogels for bone grafting and bone regeneration applications
A wide variety of bone replacement materials were employed in the past decades including metals and alloys to overcome the drawbacks of autograft and allograft. Although these materials are biocompatible, the differences between physical properties of metals and bone are still remained as a problem, resulting in the high risk of bone fracture. Currently, BCP (the mixture of HAP and b-tricalcium phosphate) has attracted much attention owning to its biocompatible properties, high biological activity as well as containing mineral components that correlate closely with bone. In addition, BCP can slowly dissolve in body, release calcium and phosphate ions, which are beneficial for the natural formation and development of bone. However, it has been difficult to shape BCPs as they are in powder form. Hence, in recent attempts to create various shapes with porous structure, composite hydrogel systems of biopolymer and BCP have been investigated. Nevertheless, the rapid biodegradation rates of the biopolymer are not included in the range acquired for biodegradable biomaterials. This limitation can be overcome by the modification of biopolymer to control the biodegradation behaviour, which increases the stability and bioactivity of the composite [29].
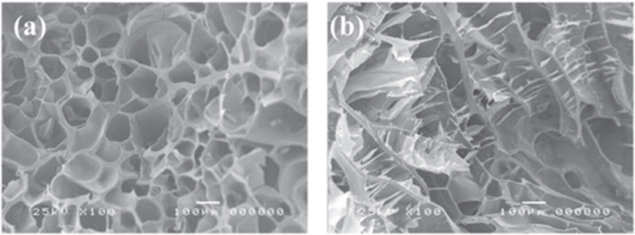
In a previous work, we prepared composite hydrogel systems consisting of oxidized alginate/gelatin/BCP via the Schiff-base reaction to mimic the microenvironment for bone regeneration. The SEM images (figure 12) showed that the morphology of the obtained hydrogel system was highly porous with the presence of unique channelized microstructures. These channels have great functional importance for the communication and transport of cells and nutrients [28].
Figure 12. SEM images of oxidized alginate/gelatin/BCP hydrogel composite (a) transverse direction and (b) longitudinal direction [28].
Download figure:
Standard image High-resolution imageIn another experiment, we have synthesized a series of hydrogel composites consisting of biopolymer (e.g. gelatin, alginate, oxidized alginate (Ox.alginate), chitosan and oxidized chitosan (Ox.chitosan) and BCP at different ratios [44]. According to the physicochemical properties of hydrogel composites including highly porous with unique channelized microstructures, degradation behaviour, biocompatible properties and the osteoconductive ability, the TA-PEG-gelatin/BCP5%, TA-PEG-gelatin/BCP10%, glginate-Ox.gelatin/BCP10%, alginate-Ox.gelatin/BCP30%, TA-Te-chitosan/BCP5%, TA-Te-chitosan/BCP10%, TA-PEG-chitosan/BCP5%, TA-PEG-chitosan/BCP10%, Ox.chitosan-PEG-TA/BCP5% and the TA-PEG-Ox.chitosan/BCP10% hydrogel composites may provide a potential application in bone grafting and regeneration, whereas Ox.alginate-gelatin/BCP10%, Ox.alginate-gelatin/BCP30%, HPA-chitosan/BCP5% and HPA-chitosan/BCP10% are likely to take advantage in regards to transplantation and cartilage regeneration. The bone tissue regeneration and repair capability of alginate.Ox-gelatin/BCP10% were tested in rabbits (figure 13). These experiments were performed at Pham Ngoc Thach Medical University.
Figure 13. Representative images of bone grafting surgery.
Download figure:
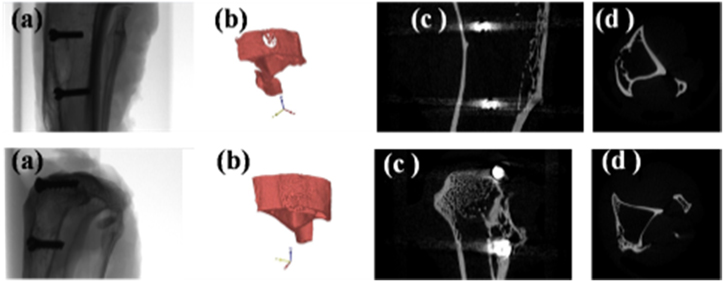
Standard image High-resolution imageMicro-CT images are shown in figure 14, in which the new bone formations are not completely filled up with the boreholes. It was displayed in control sample after 4 months of implantation (upper) while the new bone was formed with the full of boreholes in the hydrogel composite sample (lower).
Figure 14. Micro-CT images of control sample (upper) and of hydrogel composite (lower): (a) bone, (b) three dimensional surface, (c) vertical cross-section and (d) horizontal cross-section.
Download figure:
Standard image High-resolution image5. Conclusion
In this research, several synthetic and modification methods have been used to improve the therapeutic efficacy of dendrimers, hydrogels, and hydrogel composites by addressing limitations associated with traditional therapeutic treatments. They have been implicated in many biomedical applications. Moreover, the combinations of different nanomaterials or among these nanomaterials and anticancer agents were also demonstrated. At the present time, the research on biocompatible nanomaterials for use in biomedicine is very active. In particular, the authors of references [45–53] have obtained new interesting scientific results on related topics.
Acknowledgments
This review was financially supported by a research grant from Vietnam Academy of Science and Technology. Sincere thanks are to Academician Nguyen Van Hieu for his encouragement.