Abstract
The reaction-induced deformation and its recovery process in the nanometer scale of a photo-cross-linkable poly(ethyl acrylate) were in situ studied by using Mach–Zehnder interferometry. The ON–OFF modulated irradiation using two different UV wavelengths, 365 nm and 297 nm, was carried out to elucidate the effects of the reversible cross-link on the deformation kinetics of the polymer. It was found that the irradiation time-dependence of the crosslink density generated during the ON–OFF irradiation process deviates from the data obtained by continuous irradiation process, revealing the relaxation effect of the forming poly(ethyl acrylate) networks in the dark during each irradiation cycle. Furthermore, the recovery of the sample thickness can be achieved by photodissociation of the anthracene photodimers by irradiating the cross-linked sample with 297 nm UV light. These experimental results reveal the possibility of reversible control the shrinkage and swelling proceses of a polymeric film by using the photodimerization of anthracene.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Materials exhibiting reversible changes in their properties have been intensively studied in order to construct and design systems with stimuli-response behavior [1–3]. For example, swelling behavior of polymer networks driven by the protonation/deprotonation of methacrylic acid groups under UV light irradiation was studied by dynamic light scattering (DLS) [4]. Reversible change between hydrophobic and hydrophilic states of a surface with microstructures was demonstrated by taking advantage of collective nanoscale motions of DNA triggered by a change in pH [5].
Several research groups have also indicated that anthracene and its derivatives can undergo photodimerization upon irradiation with ultraviolet (UV) of wavelengths longer than 350 nm. The resulting photodimer formed by 365 nm can be reversed to its original monomers by photo-dissociation reaction upon exposure to UV light with wavelengths shorter than 300 nm [6, 7]. Reversible photodimerization of anthracene and its derivatives have been demonstrated for image recording systems [8] and can be utilized for construction of stimuli-response systems. This versatile reaction has been suggested for utilization in optical molecular switching [9], elastomeric freely suspended membranes generated by Langmuir–Blodgett membranes [10] and biosensors [11]. In addition, this reversible photo-crosslinking reactions of anthracene have been applied to reversibly control the swellability of polymer hydrogel systems [12].
We have utilized photodimerization of anthracene to control the morphology of polymer mixtures developing during the phase separation process [13, 14]. By UV photopolymerization we were also successful in generating and controlling the droplet two-phase structures [15] as well as the bi-continuous morphologies [16] of polymer mixtures at different stages of the phase separation process. In principle, freezing the ongoing phase separation process can be performed by increasing viscosity of the mixture by either photo-cross-linking or photopolymerization reactions. On the other hand, by inducing photo-dissociation of anthracene dimers using UV light with shorter wavelengths, we have experimentally demonstrated that the dissociation of photodimers in polymer mixtures can take place under irradiation with 298 nm UV light, leading to re-dissolution of the phase separated polymer blends. However, the complete dissociation of anthracene dimer was not possible because of the presence of the interfaces between different phases and in particular the spectral overlap between anthracene and its photodimer in the short wavelengths region [17].
From the viewpoint of polymer processing, most polymers undergo shrinkage upon cross-link due to the change in density accompanying the decrease in segmental free volumes induced by the cross-linking reaction. This volume change is more significant as liquid monomer is converted into solid polymer during the polymerization process. Therefore, the reaction-induced shrinkage plays an important role in the materials processing at small scales utilizing photo-polymerization and other photochemical reactions, particularly in restorative materials [18]. In order to monitor the local deformation associated with the photo-polymerization and photo-cross-link reactions in polymer mixtures, we have developed a Mach–Zehnder interferometer (MZI) system for the in situ detection of local deformation under curing with UV light [19]. We have also observed and analyzed the local deformation induced by photodimerization of anthracene in photo-cross-linked polymers and were successful in correlating the reaction-induced shrinkage and phase separation kinetics of multicomponent polymers [17]. It was found that the effect of shrinkage on the kinetics of phase separation is significant for both the liquid mixtures containing monomer as well as polymer mixtures in the bulk state [17, 20].
In this study, to elucidate the reaction-induced shrinkage as well as to minimize the inhomogeneity generated by the cross-link reaction in the bulk state of polymer, reversible photodimerization of anthracene driven by two different UV wavelengths, 365 and 297 nm, was utilized in order to perform the reversible cross-link of an anthracene-labeled poly(ethyl acrylate) (PEA-A) in the bulk state. The kinetics of the reversible cross-linking reaction was monitored by UV–Vis spectrometry under various conditions. These experimental results will be discussed in conjunction with the shrinkage-and-swelling behavior observed by Mach–Zehnder interferometry under the continuous and the ON–OFF irradiation. Finally, the possibility of a photosensitive polymer system exhibiting stimuli-response behavior driven by irradiation using two different wavelengths will be discussed.
2. Experimental
2.1. Materials
Sample used in this study was an anthracene-labeled poly(ethyl acrylate) derivative (PEA-A) with the chemical structure illustrated in scheme 1. The procedure of synthesizing PEA-A was reported in detail elsewhere [20]. The weight-average molecular weight and the polydispersity of this PEA-A are  and
and  respectively as obtained by viscosity measurement and gel permeation chromatography (GPC, Shodex System 21, Tokyo) with tetrahydrofuran (THF) as a diluent. The average anthracene label content of PEA-A is 9 anthracenes/chain (0.6 mole % in equivalent) was determined by UV–Vis spectrometry (UV-1600, Shimadzu Inc., Japan). The purification of PEA-A and preparation of the PEA-A films are the same as those described in previous publication [20]. The sample thickness was adjusted to be in the range ca. 18 μm by using an aluminum (Al) spacer.
respectively as obtained by viscosity measurement and gel permeation chromatography (GPC, Shodex System 21, Tokyo) with tetrahydrofuran (THF) as a diluent. The average anthracene label content of PEA-A is 9 anthracenes/chain (0.6 mole % in equivalent) was determined by UV–Vis spectrometry (UV-1600, Shimadzu Inc., Japan). The purification of PEA-A and preparation of the PEA-A films are the same as those described in previous publication [20]. The sample thickness was adjusted to be in the range ca. 18 μm by using an aluminum (Al) spacer.
Scheme 1. Chemical structure of anthracene-labeled poly(ethyl acrylate) (PEA-A).
Download figure:
Standard image High-resolution image2.2. Instruments
The deformation of a PEA-A film under irradiation with UV light with the wavelength 365 nm was in situ monitored by using Mach–Zehnder interferometer (MZI) equipped with a high-pressure Hg–Xe lamp (350 W, Luminex, Japan). Details of the interferometer and its operation was provided elsewhere together with the data analysis using Hilbert transform [21]. In brief, the deformation of a polymer film under photocuring with UV light was calculated from the optical path length difference (OPLD) of light travelling along the test arm and the reference arm of the MZI using the following equation [19, 20]:
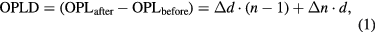
where  and
and  are respectively the optical path length observed before anf after irradiation.
are respectively the optical path length observed before anf after irradiation.  and
and  are respectively the changes in the thickness and in the refractive index of the sample before and after irradiation. From equation (1), the strain (or deformatiom) of the photocured polymer is related to this OPLD through:
are respectively the changes in the thickness and in the refractive index of the sample before and after irradiation. From equation (1), the strain (or deformatiom) of the photocured polymer is related to this OPLD through:
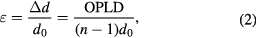
where 
 is respectively the initial thickness and the deformation (change in the sample thickness before and after the reaction) of the photo-cured sample. Here, the refractive index of the air was taken as unity. On the other hand, the change in refractive index can be independently obtained by using the condition for total reflection of the sample observed by a prism-coupler (Metricon Corp., Model 2010). Finally, the shrinkage can be obtained from equations (1) and (2) provided that the initial thickness
is respectively the initial thickness and the deformation (change in the sample thickness before and after the reaction) of the photo-cured sample. Here, the refractive index of the air was taken as unity. On the other hand, the change in refractive index can be independently obtained by using the condition for total reflection of the sample observed by a prism-coupler (Metricon Corp., Model 2010). Finally, the shrinkage can be obtained from equations (1) and (2) provided that the initial thickness  is given.
is given.
2.3. Experimental procedure
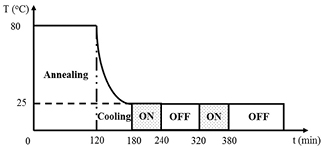
To examine the reversibility of the photodimerization of anthracene in the bulk state of polymer, a PEA-A film was first annealed at 80 °C for 120 min and was then slowly quenched to the experimental temperature at 25 °C over 60 min prior to irradiation. This treatment was necessary to eliminate the local strain generated during the course of quenching and also to erase the thermal history of the sample during the preparation step. Subsequently, the sample was irradiated under two different experimental conditions. For the first case, PEA-A was irradiated using 365 nm UV light in 60 min and the effect of the cross-link reaction on the absorption of anthracene was monitored by UV photospectroscopy. Subsequently, the absorption of anthracene moieties was monitored during the OFF time (without irradiation) for 80 min to examine the change in absorbance in the dark. This ON/OFF modulated irradiation process was repeated as illustrated in scheme 2.
Scheme 2. Thermal history and irradiation procedure for a PEA-A film in the ON–OFF modulated irradiation process.
Download figure:
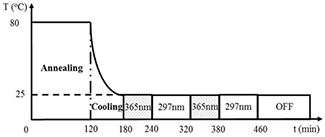
Standard image High-resolution imageFor the second experimental condition, the effects of the reversible photodimerization of anthracene on the absorption of a PEA-A film were observed under alternative irradiation using 365 and 297 nm UV light with different intensities. Namely, the PEA-A networks were constructed by irradiation with 365 nm and are subsequently dissociated by irradiation with 297 nm. For this purpose, a PEA-A film was irradiated with 365 nm UV light in 60 min, under conditions similar to the former experiments as shown in scheme 1. However, instead of turning off the excitation light, the irradiation wavelength was switched from 365 nm to 297 nm to induce the photodissociation of anthracene photodimers formed by 365 nm and thus de-crosslinking the networks within the interval of 80 min. As illustrated in scheme 3, the effects of crosslinking and de-crosslinking reaction on the deformation of PEA-A films were illustrated, as an example, for two irradiation cycles. The reaction and the deformation kinetics under these two temporal irradiation processes will be discussed for a PEA-A film in the later part of this manuscript.
Scheme 3. Thermal history and irradiation procedure for a PEA-A film in the modulated irradiation process using two different wavelengths 365 and 297 nm.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Irradiation with an ON–OFF modulation
3.1.1. Effects of irradiation intensity on the reaction kinetics.
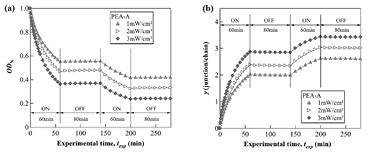
The irradiation-time dependence of the optical absorbance OD(tirr) was observed for a PEA-A film at 365 nm during the modulated irradiation process. Figure 1(a) shows the change in the normalized optical absorbance ODN(tirr) versus experimental time under three irradiation intensities I = 1, 2 and 3 mW cm−2. Here, the experimental time is defined as the sum of ON and OFF-time, i.e. the total elapse time of the experiment. It was found that the photodimerization kinetics of anthracene can be well fitted to the modified Kohlrausch–Williams–Watts (KWW) stretched exponential function [22] during the irradiation time interval:
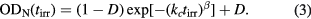
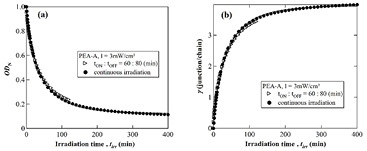
Figure 1. Photo-cross-link kinetics observed for a PEA-A film irradiated under different intensities: (a) normalized absorbance ODN, (b) crosslink density γ. Experimental time is defined as the total time required for the experiment, i.e. (tON + tOFF).
Download figure:
Standard image High-resolution imageHere ODN(tirr) is the normalized absorbance defined as OD(tirr)/OD0 where OD0 is the initial absorbance of the sample. It should be noted that OD0 is less than unity for all the samples used in this experiment. D is the baseline of the decay, expressing the limitation of the crosslink reaction under the experimental conditions using 365 nm light. kc and β are respectively the average reaction rate and the inhomogeneity index of the cross-link process.
On the other hand, it is worth noting that the optical absorbance did not change during the OFF time intervals. The crosslink density generated during the photopolymerization was calculated by using the following equation:
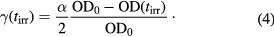
In the above equation, the numerical factor 1/2 on the right hand side arises from the fact that two anthracenes moieties form one crosslink function by the photodimerization. During the irradiation time interval, the time-evolution of crosslink density γ(tirr) was found to be also well fitted to the modified KWW stretched exponential function [22]. Furthermore, the crosslink density was unchanged during the OFF-time interval.
On the other hand, as shown in figure 1(b), the crosslink density  increases during the irradiation-time intervals and remains constant during the OFF time, indicating that the PEA-A sample is apparently under equilibrium when the light is turned off. Since the rise of cross-linking density γ with irradiation time is limited by the ON–OFF irradiation time, the full rising process cannot be analyzed by fitting to a model function. As a consequence, the initial slope defined as
increases during the irradiation-time intervals and remains constant during the OFF time, indicating that the PEA-A sample is apparently under equilibrium when the light is turned off. Since the rise of cross-linking density γ with irradiation time is limited by the ON–OFF irradiation time, the full rising process cannot be analyzed by fitting to a model function. As a consequence, the initial slope defined as  at tirr → 0 was used as a measure for the cross-link rate of each irradiation cycle.
at tirr → 0 was used as a measure for the cross-link rate of each irradiation cycle.
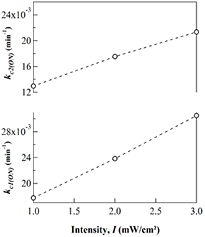
In addition, figure 2 shows that the crosslink rates obtained for two successive irradiation cycles increases with increasing the irradiation intensity. The results are consistent with the data obtained for a PEA-A under continuous irradiation with three intensities 1, 2 and 3 mW cm−2 as illustrated in figure 3. For further understanding, the cross-linking kinetics of a PEA-A under ON–OFF modulated irradiation was compared with those monitored under continuous irradiation. It was found that the OFF-time interval does not modify the absorbance and the crosslink density of the sample. The normalized absorbance  and the cross-link density
and the cross-link density  are respectively replotted versus the irradiation time tirr in figure 3. Obviously, both the reaction yield and the cross-link density increases with increasing the irradiation intensity.
are respectively replotted versus the irradiation time tirr in figure 3. Obviously, both the reaction yield and the cross-link density increases with increasing the irradiation intensity.
Figure 2. Cross-link rate of the two irradiation cycles obtained by curve fitting process using the modified KWW equation for a PEA-A film irradiated under various light intensities.
Download figure:
Standard image High-resolution imageFigure 3. Reaction and cross-link kinetics observed for a PEA-A film photo-crosslinked under UV irradiation with different intensities: (a) normalized absorbance ODN, (b) crosslink density γ.
Download figure:
Standard image High-resolution imageFor comparison, figure 4 shows the change in the normalized absorbance of anthracene ODN (figure 4(a)) and in the crosslink density γ (figure 4(b)) under continuous and ON–OFF modulation versus irradiation time. It was found that the behavior of the reaction kinetics corresponding the ON–OFF irradiation was similar to that of the continuous irradiation. In addition, the dependence of the crosslink density on irradiation time could also be fitted to the modified KWW stretched exponential function [22]. It can be seen from figure 4 that the normalized optical density ODN and the crosslink density γ obtained by the ON–OFF modulation was slightly deviated from those obtained under continuous irradiation. Probably for such stimuli (reaction) and response (deformation) systems, the difference in the magnitude of the deviation observed for these two cases (continuous versus modulated) could be observed due to the deformation of the samples occurring during the cross-linking reaction. The details will be discussed in the following section.
Figure 4. Reaction and cross-link kinetics observed for a PEA-A film under (•) continuous irradiation, (▷) modulated irradiation: (a) normalized absorbance ODN, (b) crosslink density γ.
Download figure:
Standard image High-resolution image3.1.2. Effects of irradiation intensity on the deformation kinetics.
For our curing conditions, it was found from the experiments with prism coupler that the change in refractive-index associated with photodimerization of anthracene in PEA matrix is negligibly small (Δn ⩽ 5 × 10−4). As an approximation, the strain ε is directly proportional to the change in the optical path length difference (OPLD) [19]
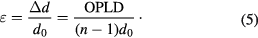
From the negligible change in refractive index before and after the photo-crosslinking reaction, the optical path length difference can be approximately expressed by the following equation:
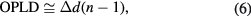
where

Here, d0 and d are respectively the thickness of the blend before and after the photo-crosslink reaction. On the other hand, n and 1 are respectively the refractive index of the sample and of the air.
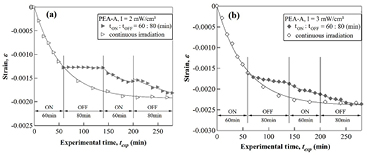
For comparison, the time-evolution of strain (shrinkage) is plotted versus the experimental time texp in figures 5(a) and (b). It is worth noting that texp here is defined as the total of the ON- and OFF-time of the experiment. As a PEA-A film was irradiated with the intensity I = 2 mW cm−2, two different relaxation behaviors were observed during the OFF-time interval. As illustrated in figure 5(a), a PEA-A film gradually shrinks with irradiation time upon irradiation. After irradiation over 60 min with the intensity 2 mW cm−2, the sample reveals a crosslink density of γ = 2.4 (junctions/chain). Subsequently, the excitation light was turned off and the shrinkage of the PEA-A film was continuously monitored during 80 min in the dark. It was found that the deformation of the PEA-A sample was not changed during this dark period as shown in figure 5(a). In the second irradiation cycle under the same conditions, the sample exhibits shrinkage again during 60 min of additional irradiation. However, unlike the first photo-cross-link step, the cross-linked sample continues shrinking during 80 min of OFF-time in the dark.
Figure 5. Time-evolution of the deformation (strain) monitored by MZI for a PEA-A film under continuous and temporal modulation using two intensities: (a) I = 2 mW cm−2; (b) I = 3 mW cm−2.
Download figure:
Standard image High-resolution imageAfter 60 min of irradiation of the second irradiation cycle, the crosslink density was γ = 3.0 (junctions/chain) was built into the sample. On the other hand, under higher light intensity I = 3 mW cm−2, the sample continued shrinking in the dark corresponding to the OFF-time interval of the first cycle. The crosslink density generated after the first and the second irradiation cycles under this light intensity is ca. γ = 3.0 and γ = 3.4 (junctions/chain), respectively. Compared to the experimental data obtained under similar conditions for the same PEA-A sample reported previously, the PEA-A sample already enters the glassy state after the first cycle of irradiation under this particular light intensity, revealing the typical behavior of amorphous polymers undergoing physical aging induced by photo-crosslinking reaction [20].
3.2. Irradiation with using two different wavelengths
3.2.1. Effects of irradiation intensity on the reversible reaction kinetics.
Similar to the temporal modulation experiments using the ON- and OFF-time intervals, the photodimerization process of anthracene, i.e. the photo-cross-linking reaction, driven by two different wavelengths 365 and 298 nm for a PEA-A film was examined by following the irradiation time-dependence of the absorbance of anthracene.
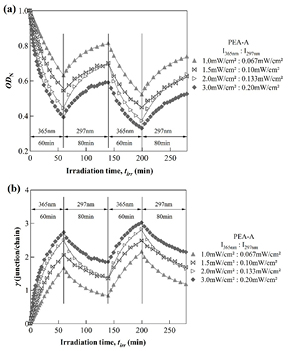
As illustrated in figure 6(a), upon irradiation with 365 nm UV light, the normalized absorption ODN decreases with irradiation time, indicating that anthracene groups attached on PEA chain undergoes photodimerization, resulting in the PEA-A networks.
Figure 6. Cross-linking kinetics (a) and the evolution of the cross-link density with irradiation time (b) obtained by modulated irradiation using two wavelengths 365 and 297 nm with different pairs of intensities.
Download figure:
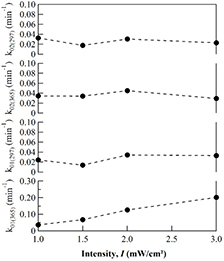
Standard image High-resolution imageAfter 60 min of irradiation, the exciting light was switched from 365 nm to 297 nm to induce the photodissociation of the anthracene photodimers. It was found that the normalized ODN rises up due to the regeneration of anthracene monomers in the sample. As shown in figure 6(a), similar recovery process was observed as the sample was irradiated with several irradiation intensities ranging from 1.0 to 3.0 mW cm−2. It should be noted that since the same Hg–Xe light source was used for irradiation, the intensity ratio at the two wavelengths 365 nm and 297 nm was determined by the wavelength distribution of the light source spectra. Namely, once the intensity at 365 nm was determined, the intensity at 297 nm is automatically obtained from the intensity sdistribution of the Hg–Xe lamp. As shown in figure 7, upon irradiation with 365 nm, stronger intensity leads to higher crosslink rate, i.e. higher rate of networking. Similarly, the stronger 297 nm intensity results in the higher de-crosslink rate, i.e. higher rate of the network dissociation. It is worth noting that the rate of the reactions was estimated by using the initial slope of the changes in absorbance of anthracene with irradiation time as previously described for the data shown in figure 2. From these experiments, it was found that the rate of crosslink and de-crosslink reactions increases with increasing the irradiation intensity. During the reaction, the residual anthracene monomer decreases with time, leading to a decrease in mobility of the system because of the network formation. As a result, the reaction rate in the second cycle is slower compared to the first one. For example, under irradiation with the intensity I365 nm = 3.0 mW cm−2, the crosslink rate in the second cycle is about 15% compared to that in the first one.
Figure 7. Reaction rates obtained by moduated irradiation using two wavelengths 365 and 297 nm with different intensities obtaind for a PEA-A film. The subscript of the reaction rate k indicates the cycle of irradiation.
Download figure:
Standard image High-resolution image3.2.2. Effects of irradiation intensity on the reversible deformation of a PEA-A film.
The effects of the reversible photodimerization of anthracene on the deformation process were in situ monitored by Mach–Zehnder interferometry for a PEA-A film at 25 °C. The dependence was observed for various irradiation intensities of the two different wavelengths, 365 nm and 297 nm of UV light. Figure 8 reveals a decrease in the thickness of a PEA-A film caused by the photodimerization of anthracene under two sets of light intensity. In the ON–OFF modulation experiments mentioned above, the sample could continue shrinking after the cross-linking reaction was terminated. This particular behavior is observed when the cross-linked PEA-A enters the glassy state due to the physical aging phenomena. For the two sets of light intensities at two wavelengths 365 nm and 297 nm, the driven shrinkage/swelling behavior is different at the initial stage of irradiation. However, as the cross-link/decross-link cycle is repeated, the repeating shrinkage/swelling behavior tends to approach to each other and merge into one at long experimental time. This particular behavior is determined by the equilibrium of the photodimerization and photodissociation (cross-link and de-cross-link reactions of anthracene). In other words, the regeneration of anthracene monomers in the sample led to the recovery in the thickness of a PEA-A film, i.e. the induced swelling. These experimental results shown here suggest that reversible dimerization of anthracene can be used to reversibly control the swelling and shrinking process of a polymer film in the nanometer scales by using light irradiation.
Figure 8. Reversible deformation (shrinkage/swelling) of a PEA-A film observed under modulated irradiation using two wavelengths 365 and 297 nm with different intensities.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, by the combination of UV–Vis spectroscopy and Mach–Zehnder interferometry, we could observe the effects of the crosslinking reaction not only on the sample shrinkage in the nanometer scales, but also on the physical aging process of a photo-cross-linked PEA-A film. The crosslink density was constant during the OFF-time interval of the ON–OFF modulated irradiation. On the other hand, in the modulated irradiation process using two different wavelengths, the regeneration of anthracene monomers from their photodimers in the PEA-A samples leads to the recovery of the PEA-A film thickness. This behavior reveals the possibility of reversible control of the swelling/shrinking process of polymer thin films in the nanometer scales by using two different wavelengths of the excitation light. These experimental results would suggest a useful method to reversibly control polymer adhesion and/or coating in the nanometer scales.
Acknowledgment
The financial support from the Ministry of Education, Japan (MONKASHO) via the Scientific Research Type B (No. 20350107) and Research-on-Priority-Area Soft Matter Physics (No. 21015018) is greatly acknowledged. D-T V-P greatly appreciates the Scholarship from the Ministry of Education (MONKASHO), Japan to pursue the PhD program at Kyoto Institute of Technology, Japan.