Abstract
Organometallic chemistry plays an increasing role in the synthesis of nanoparticles, as it provides a reliable access to metal nanoparticles with efficient control over their morphology, organization and surface chemistry. In case of magnetic nanoparticles, the synthetic tools provided by organometallic chemistry allow access to nanomaterials of high magnetization, meaning that no dead surface magnetic layer is observed. These objects are thus good candidates to be used as building blocks in composite materials of high added value. This paper reports on the organometallic synthesis of composites made of cobalt nanoparticles and carbon nanotubes. TEM investigations show that attachment of cobalt spheres and rods along the carbon nanotubes is achieved, the rods and tube long axis being aligned parallel to one another.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Magnetic nanomaterials find applications in fields as diverse as medicine, catalysis, microelectronics and energy. Depending on the application envisaged specific parameters such as magnetic anisotropy, and magnetization must be adjusted. Theoretical calculations predict an enhancement of magnetic properties when coupling magnetic nanoparticles to graphene or carbon nanotubes (CNTs) as a consequence of the intrinsic magnetism of these carbonaceous materials [1–7]. CNTs have interesting electrical [8–11] and mechanical [12] properties. They are chemically stable and present a large specific surface area. Therefore they should offer an adequate interface with a magnetic material, with the additional advantage of directing the formation of 1D hybrid materials of high magnetic anisotropy. On the other hand, organometallic chemistry affords magnetic nanoparticles with unaltered surface magnetism [13], which make them ideal candidates to study the interplay between magnetic nanoparticles and CNTs as the effect will essentially rely on surface interactions or on the interface established in between. The key to preserve surface magnetism lies in the choice of the metal precursor and reduction method, so that the by-products of the synthesis do not coordinate at the surface of the nanoparticles or coordinate via σ-donating functional groups only [14, 15]. This was well demonstrated in the case of cobalt. Cobalt nanospheres less than 5 nm in diameter and with magnetization above or close to the bulk value were obtained in perfect agreement with theoretical calculations [16]. Moreover, a careful choice of the composition of the growth medium allowed the formation of cobalt nano-disks [17, 18], rods [19] or wires [20], thus allowing a fine tuning of the magnetic anisotropy. Based on this expertise, we investigated the preparation of cobalt-CNT composites (CNT@Co) by direct decomposition of an organometallic cobalt complex, [Co(η4-C8H12) (η3-C8H13)], in the presence of suspended CNTs. Hydrogenation of this complex can be performed at room temperature which can favor attachment of cobalt onto the CNT and limit Ostwald ripening of the cobalt nanoparticles at the surface of the CNTs, which was otherwise observed when working at elevated temperatures (400 °C) [21]. Upon hydrogenation only cobalt atoms and cyclooctane, a non-coordinating molecule, are formed in the reaction medium. When working in an inert solvent, the cobalt atoms produced are virtually naked, which renders this process close to the organometallic chemical vapor deposition (OMCVD) one, and consequently offers an alternative to electron beam evaporation [22], thermal evaporation [23], or electrodeposition [24]. For comparison purposes we also investigated the attachment of preformed Co nanoparticles made by organometallic chemistry from the same cobalt precursor onto the CNTs surface. The final objective was to obtain a magnetic material the properties of which could compete with those of nowadays permanent magnets but at a lower cost.
2. Experimental
2.1. Carbon nanotubes
Pristine multi-walled carbon nanotubes (MWCNTs) 15 ± 5 nm in diameter and 5–10 µm long were provided by Nanolab Inc. Single-walled carbon nanotubes (SWCNTs) were obtained from Unidym, California. Oxidation of the CNTs (o-CNTs) was performed according to the following procedure adapted from [25]. Typically 200 mg of MWCNTs were sonicated for 4 h in 200 ml of a mixture of H2SO4/HNO3 (3:1). The sample was then washed with dilute NaOH aqueous solution and consequently with water by three centrifugation/redispersion cycles. The final solution was dried under vacuum for 20 h to remove any presence of water and adsorbed dioxygen from the surface of the CNTs. Functionalization of CNTs with oleylamine was performed according to the process reported in [26]. Typically 2 mg of CNT and 10 µl of oleylamine were sonicated 45 min in 10 ml of toluene. CNT were recovered by centrifugation (5500 rpm, 25 min, 20 °C), washed three times with ethanol to remove excess oleylamine, dried under vacuum and redispersed in toluene to reach a 1 mg ml−1 concentration.
2.2. Co nanoparticles
Co nanoparticles were prepared according to already published procedures [17, 19]. Co spheres with mean size of 3 ± 0.5 nm and rods of aspect ratio 3 (24 ± 7 nm long, 8 ± 2 nm large) were selected for the preparation of the CNT@Co composites.
2.3. CNT@Co. direct route
The direct decomposition of the cobalt precursor [Co(η4-C8H12) (η3-C8H13)] onto CNTs was performed employing 5 mg of CNT and different amounts of precursor (adjusting the Co precursor/CNTs weight ratio to 42, 62, 71 and 84%) dispersed in 20 ml of tetrahydrofuran (THF) or pentane, the solution was sonicated for 30 min and stirred 20 h under 3 bars of dihydrogen. The resulting nanocomposites (CNT@Co) were isolated using a magnet and dried under vacuum.
2.4. CNT@Co. two-step procedure
100 µl of the toluene solution of CNT functionalized by oleylamine are dispersed in 800 to 870 ml of toluene under sonication for 45 min. Then 30, 77, 100 or 150 µl of a 0.01 mg ml−1 solution of cobalt nanoparticles in toluene were introduced and the mixture was placed in an ultrasound bath for 30 min. The resulting nanocomposites (CNT@Co) were isolated using a magnet and dried under vacuum.
2.5. Characterization
Samples were characterized by TEM using a JEOL JEM 1011 microscope operating at 100 kV at the TEMSCAN facility of University Paul-Sabatier.
3. Results and discussion
Two strategies were envisaged to form CNT@Co composites. The first one consisted in generating Co atoms in solution, from an organometallic precursor directly in the presence of suspended CNTs (direct route). The second one consisted in a two-step procedure, the synthesis of Co nanoparticles and then their grafting onto the CNTs (two-step procedure).
3.1. Direct route
The idea behind this first approach is to use CNT as a stabilizing agent for the growing nanoparticles. Indeed, arene groups from flexible surface ligands have already been reported to interact with the surface of metal nanoparticles and participate in their stabilization [27–29]. The relatively large diameter of the CNTs insures that the growing Co nanoparticles will locally encounter a flat graphene-like surface. Furthermore, the lattice parameters of graphene and hcp cobalt (0 0 1) are 2.46 and 2.50 Å, respectively, and in both cases the structure is composed by atoms arrayed hexagonally [30]. These similarities imply a < 2% mismatch that should enable the establishment of a stable interphase between metallic cobalt and the CNTs. Theoretical calculations on a similar system have predicted the two possible stable configurations included in figure 1(b), considering two layers of cobalt atoms [31–34]. The two configurations rely on locating the first layer of cobalt atoms directly on top of the carbon atoms (top-fcc) or shifted to bridge positions (bridge-top). The effective overlapping of the π bond (sp2 hybridization) of the carbon atoms with the 3d orbital of cobalt in the top-fcc configuration proposed would favor the sought interface between the metallic cobalt and the CNTs. The overlapping in the bridge-top configuration will be less favored because of the increased distance between Co and C atoms.
Figure 1. Schematic representations (a) of the reaction undergone by the cobalt precursor [Co(η3-C8H13)(η4-C8H12)] under 3 bars of H2 to obtain cobalt atoms and (b) of the more stable adsorption geometries of Co (0 0 1) on a graphene sheet (the blue color corresponds to Co atoms in the first layer while the red corresponds to the second one).
Download figure:
Standard image High-resolution imageFollowing this idea, we selected [Co(η4-C8H12) (η3-C8H13)] as cobalt precursor. Hydrogenation of this complex can be performed at room temperature, favoring the attachment of cobalt onto the CNT. Upon hydrogenation only cobalt atoms and cyclooctane, a non-coordinating molecule, are formed in the reaction medium, as depicted in figure 1(a). When working in an inert solvent, such as pentane, the cobalt atoms produced are virtually naked and accordingly, this situation should enforce the interaction between Co and CNTs. The cobalt precursor/CNT weight ratio was varied in a way to optimize this interaction and favor the formation of Co nanoparticles directly onto the CNT surface.
In figure 2(a), a typical TEM image of the optimized nanomaterial is reported. No morphology control was achieved. In these conditions, Co nanoparticles nucleate and grow in the reacting medium before reaching the surface of CNTs. In addition, the large cobalt particles observed can be a consequence of an Ostwald ripening of first formed cobalt nanoparticles at the CNT surface [35].
Figure 2. TEM image of a CNT@Co nanocomposite obtained by hydrogenation of [Co(η4-C8H12) (η3-C8H13)] in pentane (a) or THF (b). Pristine MWCNTs. Optimized conditions were respectively obtained for Co/CNT weight ratios of 62 and 84%.
Download figure:
Standard image High-resolution imageTo prevent this, we performed the same reaction in THF. This is a weakly coordinating solvent often used to temporarily stabilize metal nanopowders when synthesized by the solvated atom approach [36]. A typical image of the nanomaterial thus obtained, is depicted in figure 2(b). Very small Co crystallites with a diameter less than 5 nm appear attached along the CNT surface, supporting the hypothesis related to the presence of THF, that is, that this solvent can limit the coalescence of the nanoparticles. In this regard, solvent molecules stabilize the Co nanoparticles formed in solution, allowing them to attach onto the CNT surface before any coalescence can take place. We can consider the driving force for the attachment to be the stronger interaction of the C6 hexagonal carbon units of the CNT with Co, compared to the THF/Co interaction. Indeed, a control experiment carried out in the absence of CNT displays only large chunks of cobalt supporting the crucial role played by the CNT surface in the stabilization of the nanoparticles. Alternatively, THF molecules can stabilize intermediate surface organometallic clusters formed along the surface of the CNTs, as already observed and discussed in the case of Cr carbonyl surface complexes [37], which then migrate and nucleate nanoparticles directly at the surface of the CNTs. However, the distribution along the CNT is not homogeneous and a larger view of the system still reveals the presence of large agglomerates such as those observed when the reaction was carried out in pentane. So, even if THF improves the control over nucleation and growth of nanoparticles, it is not efficient enough to totally prevent their coalescence: some nanoparticles interact with the CNT surface, which stabilizes them, while others form uncontrolled aggregates upon coalescence.
Interestingly, the cobalt nanoparticles attach on the outer graphitic surface of the CNT and not on the inner one, which is in agreement with the higher reactivity of the graphitic outer layer over the inner one [38].
It is noteworthy that these experiments were carried out on pristine CNTs. To reinforce the interaction between Co nanocrystals and CNTs, partial oxidation of the CNTs was envisaged. The tubes were submitted to an acidic treatment to generate surface carboxylic acid groups. As these functional groups are well known to stabilize Co nanoparticles, the idea behind was to drive the coordination of the first nuclei of Co formed onto the CNTs more efficiently than when only aromatic C6 units are available for coordination. Figure 3 shows that in this case, the CNTs@Co composites indeed display a homogeneous overlayer of Co nanoparticles onto the CNTs surface, which can be reproducibly obtained. The diameter of these Co nanoparticles lies below 5 nm, which is the critical value under which size effects such as enhanced magnetization and anisotropy are expected, and were previously observed in Co nanoparticles [16].
Figure 3. TEM image of a CNT@Co nanocomposite obtained by hydrogenation of [Co(η4-C8H12) (η3-C8H13)] in THF. Oxidized MWCNTs. (Optimized conditions: Co/CNT weight ratio 61%).
Download figure:
Standard image High-resolution image3.2. Two-step procedure
The Co nanoparticles are prepared by hydrogenation of the same cobalt precursor, [Co(η4-C8H12) (η3-C8H13)], in the presence of oleic acid in an organic solvent, anisole, according to a published procedure (see experimental part). The size and shape of the nanoparticles depend on the Co/acid ratio and concentration of free acid in the medium as confirmed by theoretical calculations [39]. It is well known from literature results that in such conditions, the nanoparticles are stabilized by a double layer coating of oleic acid [40] as depicted in figure 4.
Figure 4. Schematic drawing of the organization of oleic acid around Co nanoparticles and possible interaction with a surfacted MWCNT.
Download figure:
Standard image High-resolution imageIn these conditions, direct interaction between the nanoparticles and pristine CNT is not favored. To insure interaction between the nanoparticles and CNTs, the CNTs were first surfacted with oleylamine. This method was inspired by the paper from Li et al that focuses on the decoration of CNT with maghemite nanoparticles [26]. The kinked long chain of the oleylamine favors Van der Waals interactions with the carbon surface and leaves the amino functionality available for further interaction with the nanoparticles. Strong electrostatic interactions or hydrogen bonds are expected to settle when the oleic acid coated Co nanoparticles are mixed with the oleylamine surfacted CNTs. As expected, TEM investigation evidences small Co NPs spread along the CNTs (see figure 5).
Figure 5. Typical TEM images of CNT@Co composites obtained from oleylamine surfacted MWCNT(a) or SWCNT(b) and oleic acid coated Co NPs (Optimized conditions: Co/CNT weight ratio of 30% (a), 50% (b)).
Download figure:
Standard image High-resolution imageAnalogously, the same procedure was followed with Co nanorods as magnetic building blocks. A typical TEM image is displayed in figure 6.
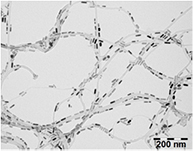
Figure 6. Typical TEM images of CNT@Co composites obtained from combining oleylamine surfacted SWCNT and oleic acid coated Co nanorods. (Optimized conditions: Co/CNT weight ratio = 50%).
Download figure:
Standard image High-resolution imageThe Co nanorods align parallel to the CNT axis. This orientation maximizes the electrostatic interaction between the overlayer of oleic acid surrounding the Co nanorods and the amine functionalities present at the surface of the surfacted CNTs. The nanomaterial thus obtained combines the morphological anisotropy of the 1D CNT and Co nanorods. Previous HRTEM investigations have evidenced that the crystalline c-axis corresponds to the long axis of the Co nanorods. From a magnetic point of view, this c-axis corresponds to the easy axis of magnetization of a magnetic Co rod of such aspect ratio (3). The alignment parallel to one another of the Co nanorod long axis and CNT axis gives therefore the optimum orientation to reach a 1D magnetic hybrid nanomaterial of large magnetic anisotropy.
4. Conclusion
We have designed a strategy to produce Co nanoparticles/CNT composites following an organometallic approach. The size of the nanoparticles, less than 5 nm, and density along the CNT are adequate to study the effect of magnetic coupling between Co and the carbon matrix. The 1D hybrid materials produced through direct hydrogenation of [Co(η4-C8H12) (η3-C8H13)] in the presence of pristine CNT is particularly well-suited as this procedure and choice of cobalt precursor ensures direct interaction between the Co nanoparticles and the surface of the CNTs. Having in mind that permanent magnets are essential in transport, energy, refrigeration and IT applications, these new materials should attract the attention of many researchers. In particular we have demonstrated that the attachment of Co nanorods was also possible, which should lead to a material of high anisotropy suitable for usage as a permanent magnet. Permanent magnets presently use critical raw materials which are increasingly sensitive to market distortions. So, the development of novel advanced magnetic material nanocomposites based on the graphenic materials will imply a substantial improvement in the industry-related and simultaneous drop down in terms of costs. In this context we are now investigating the magnetic properties of the nanomaterials described in this paper.
Acknowledgments
NF-T, MAC-D, and VS acknowledge the financial support from the Xunta de Galicia (Regional Government, Spain) under project EM2014/035, and from the Spanish Ministerio de Economía y Competitividad under project CTM2014-58481-R.
Footnotes
- *
Invited talk at 8th Int. Workshop on Advanced Materials Science and Nanotechnology (Ha Long City, Vietnam, 8–12 November 2016).