Abstract
A novel magnetically recoverable hematite nanoparticles (α-Fe2O3 NPs) was fabricated by a simple, one pot, and green method using the rhizome of Cyperus rotundus L., as a reducing and stabilizing agent. The prepared nanoparticles were well characterized by all parameters. TEM showed that the hematite nanoparticles had a rhombohedral shape and ranged in size from 80 to 100 nm. The phase study of the α-Fe2O3 nanoparticles was confirmed by Raman spectroscopy. In addition, the synthesized nanoparticles shows good photocatalytic activity in degradation of highly toxic Congo red dye within 25 min, and the same NPs exhibits higher catalytic activity for the reduction of 4-nitro-o-phenylenediamine (4-NPD) to 1,2,4-benzenetriamine in the presence of NaBH4 within 12 min. After the reaction, the catalyst was recovered and reused three times without significant loss of catalytic activity.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Noble metal nanoparticles, such as Au, Ag, Pd, Pt, and Fe, have attracted considerable interest in various fields, such as chemistry [1], physics [2], biology [3], medicine [4], and electronics, because of their unique properties that different from their bulk counterparts [5]. In particular, these metal nanoparticles (NPs) have attracted significant attention for potential applications in biomedicine [6–8], sensing [9], fuel cells [10], catalysis [11–13], labeling [14], photonics [15, 16], and electronics [17]. The synthesis of noble NPs involves the reduction of a metal salt using a range of chemical, physical, mechanical and biological methods. On the other hand, biogenic materials, such as plant extracts and microbes, were used to reduce these metal salts to NPs because they are cost effective, simple and ecofriendly. The biomolecules present in plant extracts, such as steroids, carbohydrates, flavonoids and sapogenins act as reducing agents and phyto-constituents as the capping agents, which impart stability to NPs [18].
Noble NPs can be used as catalysts for organic transformations but the separation of these NPs from the reaction mixture is difficult and remains an important issue. To overcome the above problems, many research groups have examined the preparation of magnetite nanoparticles. Recently, magnetic nanoparticles have emerged as viable, strong and readily available alternatives to common materials and are used as heterogeneous catalysts for different organic transformations [19]. The polymorphic nature of iron oxide is known for a long time, but α-Fe2O3 is the most important polymorph existing in nature as hematite. Hematite has a rhombohedrally centered hexagonal structure with a close-packed oxygen lattice [20] and it is believed to be a specific candidate for applications, such as sensors, catalysts, data storage materials, fine ceramics, pigments, and photoelectrochemical cells [21–24].
In addition, hematite nanoparticles can be separated from the reaction mixture using an external magnetic field and reused for further reactions as a sustainable point of view [25]. Therefore, several methods have been employed to produce durable and reusable heterogeneous catalysts for the reduction of nitro compounds. Among the various transition metal oxides, α-Fe2O3 nanoparticles (α-Fe2O3 NPs) are more stable in aqueous solutions and high magnetic properties [26, 27].
Colored dyes have been used in many industries like food, textile, paper, pharmaceuticals and cosmetics. Among these, most of the dyes are not easily degradable due to its complex aromatic structure. Congo red (CR) is a synthetic dye with two azo chromophores which makes them mutagenic and carcinogenic. Therefore, it is desirable to removal of such toxic coloured agents in adequate method, although several chemical and physical methods have been used for the removal of CR dye pollutants [28–30]. Similarly, 4-nitro-o-phenylenediamine (4-NPD) has shown remarkable improvement in mutagenicity when exposed to plant enzymatic systems [31]. On the other hand, 4-NPD may pose a serious potential threat to the health and well-being of humans. Many aromatic nitro compounds are included in environment legislation. Therefore, to reduce these compounds, different nanomaterials have been developed as catalysts [32, 33]. However, biosynthetic method using plant extracts is simple and environmentally friendly compared with chemical and physical methods.
This paper proposes the green synthesis of hematite nanoparticles using the rhizome of Cyperus rotundus L. a traditional medicinal plant used widely to treat stomach ailments, wounds, boils, and blisters [34–37]. Phytochemical studies of C. rotundus revealed the presence of flavonoids, tannins, alkaloids, starch, furochromones, and glycosides [38, 39]. The above green approach is comparatively facile, fast and eco-friendly because it reduces the environmental hazards by utilizing non-toxic chemicals and renewable materials owing to their larger surface area-volume ratio. The synthesized nanoparticles were used as a catalyst for the degradation of highly toxic CR dye and the same NPs used for the reduction of 4-NPD.
2. Experimental
2.1. Materials and methods
Iron (III) chloride (FeCl3), 4-NPD and sodium borohydride were obtained from Sigma-Aldrich. The air dried rhizome of C. rotundus was obtained from local suppliers in Yeongchon, South Korea.
2.2. Preparation of C. rotundus rhizome extract
Dried C. rotundus rhizome was crushed to make a fine powder, and 5 g of it was placed in 200 ml of deionized milli-Q water. The broth solution was then boiled for 10 min, filtered through a 0.2 mm filter and stored at 4 °C until needed.
2.3. Synthesis of α-Fe2O3 nanoparticles
Initially, 5 ml sample of C. rotundus extract was added to 50 ml of the prepared 2 mM iron (III) chloride (FeCl3) solutions and sonicated for 2 h at 60 °C. The resulting nanoparticles were purified by centrifugation in a Beckman Coulter's Avanti J-E centrifuge (USA) at 10 000 rpm for 20 min.
2.4. Characterization of α-Fe2O3 nanoparticles
The ultraviolet–visible (UV–Vis) spectra of the synthesized α-Fe2O3 NPs were recorded using a UV–Vis spectrophotometer (Optizen 3220, double beam). Fourier transform infrared (FTIR, JASCO spectrophotometer) spectroscopy was performed to determine the bioactive molecules present in the C. rotundus extract that may account for reduction and stabilization of nanoparticles. The size and morphology of the nanoparticles were analyzed by field emission scanning electron microscopy (FE-SEM coupled with an energy dispersive x-ray microanalysis (EDS) detector, Hitachi S4200, Japan) and transmission electron microscopy (TEM, FEI Tecnai G2 F20 ST FE-TEM). The chemical compositions of the α-Fe2O3 NPs were analyzed by EDS attached to the transmission electron microscope. Crystal structure identification was achieved by x-ray diffraction (XRD) using Cu-Kα radiation (PANalytical X'Pert MRD model). In addition, a phase study was performed by Raman spectroscopy using XploRA Plus, HORIBA Scientific with a TE air cooled CCD detector. The weight loss % of the nanoparticles were performed by thermogravimetric analysis (TGA) coupled with differential thermal calorimetry (DTA, SDT-Q600 V20.5 Build 15) from room temperature to 700 °C under a N2 atmosphere at a heating rate of 10 °C min−1. The magnetic properties were measured by vibrating sample magnetometry (VSM, Lake Shore Cryotronics, Inc., Idea-VSM, model 662).
2.5. Photocatalytic degradation of CR dye
The photocatalytic experiment was carried out using 15 W Ultraviolet fluorescent lamp. 100 mg of α-Fe2O3 nanoparticles were added to 200 ml aqueous solution of CR (10 mg l−1). The solution was continuously stirred in the dark for 1 h to maintain adsorption of CR onto α-Fe2O3 nanoparticles. Then the solution was exposed to 15 W ultraviolet fluorescent lamp at room temperature. Degradation of CR aqueous solution was monitored using UV–Vis spectroscopy by collecting the samples at regular intervals.
2.6. Catalytic reduction of 4-NPD
The reduction of 4-NPD was examined as a model reaction to confirm the catalytic activity of the synthesized α-Fe2O3 NPs. The catalytic reaction was monitored by UV–Vis spectroscopy. In these experiments, 2.0 ml of 0.1 mM 4-NPD was mixed with 1.0 ml of 0.1 M NaBH4 in a quartz cuvette, and the spectrum was measured. After obtaining the spectra, 3.0 mg of the hematite nanoparticles was then added to the above reaction mixture as a catalyst. After adding the α-Fe2O3 NPs, the UV–Vis absorption spectra were recorded at 40 s intervals over the scanning range, 200–600 nm, at room temperature (25 ± 2 °C).
3. Results and discussion
3.1. Characterization of α-Fe2O3 nanoparticles
The UV–visible spectra of α-Fe2O3 NPs synthesized using aqueous C. rotundus extracts showed continuous absorption in the visible region, but small differences in the displayed UV range. The α-Fe2O3 NPs showed strong absorption in the UV range compared to FeCl3 (figure 1(a)). Similar UV–visible spectra were obtained using the previously reported methods [40, 41].
Figure 1. (a) UV–Vis spectra, (b) FTIR spectra, (c) XRD patterns and (d) TGA-DSC traces of α-Fe2O3 NPs.
Download figure:
Standard image High-resolution imageFigure 1(b) shows the FTIR spectra of the as-synthesized α-Fe2O3 NPs. In the spectrum, the vibration band at 3463 cm−1 was ascribed to the O–H stretching and a vibration band at 1631 cm−1 was assigned to C=C stretching [42] due to capping by bioactive molecules present on the surface of the iron nanoparticles. The strong absorption bands at 580 and 474 cm−1 were assigned to the Fe–O group, which is typical for α-Fe2O3 [43] due to metal oxygen stretching vibration modes, and were not observed in the plant extract.
The synthesized α-Fe2O3 NPs were highly crystalline with XRD peaks corresponding to the face-centered cubic (fcc) phase of metallic iron. All the XRD peaks were strong, narrow and sharp without the presence of other impurity peaks indicating that the as-synthesized product was well crystallized. The major XRD peaks at 24.14°, 33.24°, 35.61°, 40.81°, 49.47°, 54.09°, 57.62°, 62.46°, and 64.13° 2θ were assigned to the (0 1 2), (1 0 4), (1 1 0), (1 1 3), (0 2 4), (1 1 6), (0 1 8), (2 1 4) and (3 0 0) planes, respectively (figure 1(c)). The products were indexed to pure α-Fe2O3 NPs with a rhombohedral structure, which is in agreement with the reported [21] values (hematite, JCPDS no.33-0664). The high purity indicates that the as-synthesized α-Fe2O3 NPs are good for catalytic applications. Similar XRD peaks to those reported previously were observed [44, 45].
TGA was carried out to determine if the precursor had been converted completely to the desired metal oxide from room temperature to 700 °C. The mass loss occurs in the following three steps: a weight loss of 2.5% in the first step (100 °C) due to the removal of adsorbed moisture and other volatile matter in the nanoparticles; a weight loss of 13.1% in the second step (200 °C), indicating the decomposition of capping agents and other biomolecules present in the nanoparticles; and a weight loss after 250 °C (20.1%) due to the adsorbed oxygen species [46]. The final residual mass was found to be 64.3%, which confirmed the presence of α-Fe2O3 NPs (figure 1(d)). The differential scanning calorimetry (DSC) thermogram revealed heat variations associated with an exothermic and endothermic reaction. The endothermic peaks observed at 138.5 °C and 300.7 °C, were assigned to thermal decomposition of the bioactive molecules present in the plant extract.
The surface morphology of the prepared α-Fe2O3 NPs was examined by SEM (figure 2(a)), which suggested that the particles were slightly irregular in shape and size. The porous structure formed by the network of spherical nanoparticles interlinked over a rhombohedral material [47] was also evident from the images. The TEM images of the nanoparticles showed that the particles have both spherical and rhombohedral morphologies [48] and monodisperse in nature (figure 2(b)). The mean grain size was found to be approximately 60 and 80 nm. Hematite nanoparticles with few agglomerates were visible in the TEM images [49]. The elemental composition assigned by EDS revealed a strong signal at ~0.7 and 6.2 keV for iron and 0.2 keV for oxygen (figure 2(c)) due to surface plasmon resonance. The typical absorption peaks for C and Cu was assigned to the carbon coated copper grid used for sample preparation.
Figure 2. (a) SEM images of α-Fe2O3 NPs at 1.00 µm, (b) TEM image of α-Fe2O3 NPs at 100 nm, (c) EDAX peaks of α-Fe2O3 NPs.
Download figure:
Standard image High-resolution imageTo further clarify the phase of the rhombohedral particles, figure 3 shows the Raman spectrum of α-Fe2O3 NPs. The α-Fe2O3 NPs showed three strong peaks around 222, 281, and 399 cm−1, and two weak bands at 483 and 610 cm−1. The peaks at 281 cm−1 and 399 cm−1 in the Raman spectrum of iron are related to the stretching (Fe–O) mode between two Fe and O atoms [50]. The positions and intensities of these bands are in good agreement with the previously reported data for face-centered α-Fe2O3 NPs [51, 52].
Figure 3. Raman spectra of the α-Fe2O3 nanoparticles.
Download figure:
Standard image High-resolution imageTo understand the magnetic behavior of the synthesized α-Fe2O3 NPs, magnetic hysteresis measurements were carried out in an applied magnetic field at room temperature. The values of saturation magnetization (Ms), remanent magnetization (Mr), and coercivity (Hc) are shown in figure 4(a). The magnetization increased almost linearly with increasing applied magnetic field up to 5000 Oe. The magnetization measurements of the α-Fe2O3 NPs exhibit hysteresis loops with the saturation magnetization (Ms), remanent magnetization (Mr) and coercivity (Hc) of 10.01 emu g−1, 1.03 emu g−1 and 200 Oe, respectively, suggesting that the rhombohedral α-Fe2O3 NPs are ferromagnetic in nature.
Figure 4. (a) VSM of α-Fe2O3 NPs. Time-dependent UV–Vis absorption spectra showing the reduction of 4-NPD by (b) NaBH4 only, (c) NaBH4 in the presence of α-Fe2O3 NPs, and (d) first order kinetics.
Download figure:
Standard image High-resolution image3.2. Catalytic reduction of 4-NPD using α-Fe2O3 nanoparticles
The catalytic activity was first measured using NaBH4 for the reduction of 4-NPD. The reduction reaction was monitored by UV–Vis spectroscopy. The intensity of the color of 4-NPD changed after adding an aqueous solution of NaBH4. This color change was attributed to the formation of the phenolate ion. The phenolate ion showed an absorption peak at 400 nm, which remained intact in the presence of NaBH4 for 30 min, as shown in figure 4(b). This suggests that no reduction occurred in the absence of the catalyst. This might be due to the high kinetic barrier between the mutually repelling negative ions of phenolate ion and borohydride ion [52]. After the addition of α-Fe2O3 NPs (1.0 mg ml−1), the reduction of 4-NPD was completed within 12 min, as shown in figure 4(c). The intensity of the absorption peak at 400 nm decreased and finally disappeared, giving rise to a peak at 300 nm, indicating the reduction of 4-NPD to 1,2,4-benzenetriamine. The rate constant, which was calculated by plotting ln(At/A0) as a function of time for the reduction of 4-NPD, was found to be 7.0 × 10−3 min−1, as shown in figure 4(d).
3.3. Photocatalytic degradation of CR dye using α-Fe2O3 nanoparticles
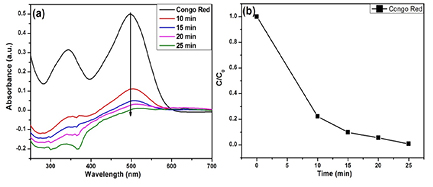
The decolorization performance of the synthesized α-Fe2O3 NPs were tested on CR dye. Figure 5(a) shows the adsorption spectra of the CR solution in the presence of α-Fe2O3 NPs at different time intervals. The main absorption peak of CR centered at 500 nm before and after irradiation. When the fluorescence lamp was turned on, the main peaks decreased with the increased irradiation time, revealing that the CR solution was decomposed in the present system within 25 min. Figure 5(b) shows the photocatalytic degradation rate of CR.
Figure 5. (a) UV–Vis spectra of the CR solution in the presence of the α-Fe2O3 irradiated by a fluorescence lamp at different time intervals, (b) photocatalysis degradation rates of CR.
Download figure:
Standard image High-resolution imageAfter the reaction, the catalyst was recovered by an external magnetic field. The residues were then washed with dichloromethane and dried at 80 °C. The recovered catalyst was then reused in the same reduction reaction under identical conditions three times without any significant loss.
4. Conclusion
This paper reported a green method for the synthesis of hematite α-Fe2O3 NPs from iron(III) chloride (FeCl3) using C. rotundus extract as a reducing agent. XRD and EDX analyses confirmed the presence of pure hematite nanoparticles without any impurities. Highly crystalline, spherical and rhombohedral nanoparticles, approximately 60–80 nm in size, were prepared. The hysteresis loop suggested that rhombohedral α-Fe2O3 NPs are ferromagnetic in nature. The synthesized nanoparticles exhibited strong catalytic activity for the degradation of CR dye by photocatalytic method and reduction of 4-NPD to 1,2,4-benzenetriamine in the presence of NaBH4. After the reaction, the catalyst was recovered and reused three times without any significant loss of catalytic activity. Considering the catalytic activity, recycling stability, and preparation method, the α-Fe2O3 NPs have potential applications in the field of catalysis.
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2014R1A2A1A11052391), the Nano Material Technology Development Program (2012M3A7B4049675) and the Ministry of Education (2014R1A6A1031189).