Abstract
In this study we report on the green synthesis of silver nanoparticles using extracts from selected morphological parts of Zanthoxylum capense. UV–vis spectra of the biosynthesised silver nanoparticles (AgNPs) revealed absorption peaks at around 450 nm, indicative of the nanoparticles' surface plasmon resonance, whilst infrared vibrational frequencies indicated the presence of flavonoids, alkaloids, and free and bonded sugars which could be responsible for the reduction and stabilisation of the AgNPs. 1H-NMR fingerprinting of the aqueous knob extract confirmed the active bio-reducing phytochemical of the knobs to be 6-O-p-coumaroyl-β-D-glucopyranoside. The nature, shape and morphology of the biosynthesised AgNPs were examined using transmission electron microscopy (TEM), selected area electron diffraction (SAED), scanning electron microscopy (SEM) and energy dispersive x-ray (EDX) analysis. Z. capense AgNPs were mostly spherical in shape with particle sizes in the range of 4–28 nm, 7–20 nm and 4–32 nm for leaves, knobs and roots, respectively. Leaf extracts were the most efficient in the synthesis of AgNPs with an average yield of 0.027 g AgNPs per g of plant (dry mass). The AgNPs were more effective than sodium hypochlorite (NaOCl) and sodium dichloroisocyanurate (NaDCC) in the control of in vitro fungal contamination in nodal explants of Z. capense up to two weeks. Shoots induced from the surface sterilised explants were further used for shoot multiplication on benzyl aminopurine (BAP) and kinetin (KIN). BAP at 0.5 mg l−1 gave the highest percentage (88.6%) of explants bearing shoots with an average of 4.78 shoots per explant. A total of 15 fungal endophyte strains associated with Z. capense were identified using molecular methods.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Research geared towards the synthesis and application of nanoscale silver particles have gained serious attention in recent years due to their unique physicochemical and biological properties. The biointeractions of silver nanoparticles (AgNPs) are significantly enhanced by their large surface area-to-volume ratio as compared to their bulk counterparts [1]. This, along with their chemical, conductive and optical properties allows them to find applications in modern medicine [2, 3], biosensing [4, 5], drug delivery [6, 7], renewable energy [8] and catalysis [9–11]. Their broadened spectrum of antifungicidal and antibactericidal properties have made them popular in consumer products like cosmetics and textiles. These properties have also enabled them to find application in food processing [1] and water purification [12].
Biocatalysed synthesis of AgNPs has been reported from various bio-resources and is an Ag1 to Ag0 reduction route that is preferred over chemical and physical approaches due to cost-effectiveness, it being less hazardous to living organisms and being environmentally friendly [13]. AgNPs have been successfully synthesised from medicinal plant species [14], leafy vegetables [15] and seaweeds [16, 17]. Other biofactories include animal tissues [18], animal waste, endophytic fungi [19, 20] and other microorganisms [21, 22]. Optimisation of these bio-inspired synthetic methods is on-going with the introduction of the microwave-assisted technique [23] and the use of the statistical experimental design tool, central composite design (CCD) which incorporates biosynthesis parameters like incubation time, concentration and volume of reaction mixture components [24]. Medicinal plants are known to be rich sources of biologically active compounds [25]. The presence of diverse secondary metabolites in addition to primary metabolic products in these plants contributes largely to their efficacy in the green synthesis of AgNPs. Since there are variations in the distribution of secondary metabolites across different morphological parts of plants, different plant parts including leaves, fruits, bark and roots are used for the synthesis of AgNPs [26–28].
Zanthoxylum capense (Z. capense) is a woody South African species of the Rutaceae family and is found in KwaZulu-Natal and the Eastern Cape and is also distributed across Mozambique and Zimbabwe. It grows to a height of about 10 m with its trunk covered in knobs [29]. The fruits possess a strong smell of citrus. The roots and stem bark of Z. capense are used traditionally for the management of snake bite, epilepsy, toothache [30] and HIV/AIDS-related diseases [31]. Amabeoku and Kinyua recently reported on the anticonvulsant potential of Z. capense leaf extracts [29]. The incessant collection by traditional healers and problematic propagation resulting from its hard seededness [32] has resulted in Z. capense being classified as a threatened species [33].
In vitro propagation can provide an avenue for the mass production of desired plant materials which can be used to produce secondary metabolites of interest [34–36]. However, asepsis plays a vital role in the establishment of in vitro protocols for either research or commercial tissue culture purposes [37]. Since fungi and bacteria rapidly proliferate in in vitro cultures [38] and also compete with plant cultures for media nutrients [39], explants for in vitro propagation must first undergo surface sterilisation using conventional sterilants such as sodium hypochlorite, calcium hypochlorite, ethanol or mercuric chloride at varying concentrations and soaking durations [40, 41] while antibiotics and fungicides are sometimes incorporated into the culture media [42]. But commonly practiced surface sterilisation techniques are not very effective in woody plant species [43]. Moreover, it has been reported that antibiotics can be phytotoxic, inhibiting in vitro shoot multiplication and plantlet regeneration [44, 45]. Silver nanoparticles have recently emerged as more efficient and less toxic (to humans during handling) for the control of in vitro microbial contaminants owing to their multidimensional modes of microbial inhibitory action [46]. Nanosilver-mediated elimination of contamination has been reported in some plant species [47–50].
To the best of our knowledge, there are only two reports on micropropagation in the genus Zanthoxylum [41, 51]. This lack of literature may be associated with the high level of fungal or bacterial contamination in culture. In addition, no Zanthoxylum species have been studied for their potential in the green synthesis of AgNPs or the use of the synthesised nanoparticles in controlling in vitro microbial contamination. The present study, therefore, aimed to explore the rich phytoconstituents of Z. capense as bioreductants, capping and stabilising agents in the synthesis of AgNPs, which in turn were tested for the management of microbial contamination during in vitro culture. In addition, endophytic fungi associated with Z. capense during in vitro culture were isolated and identified.
2. Materials and methods
2.1. Collection of plant materials
The leaves, knobs and roots of Z. capense were collected in June 2015 from the University of KwaZulu-Natal (UKZN), Durban, South Africa. The plant was identified by Dr Syd Ramdhani and a voucher specimen was deposited in the Ward Herbarium (No. Bodede 01) at UKZN, School of Life Sciences.
2.2. Preparation of plant extracts
The leaves, knobs and roots of Z. capense were air dried for four weeks and ground to near-powder using a metallic mortar and pestle. Each plant part (5 g) was boiled with 100 ml of Millipore™ water for 15 min and the resulting aqueous solutions were filtered through Whatman no. 1 filter paper. The crude aqueous extracts were stored at 4 °C and used within 48 h.
2.3. Synthesis of silver nanoparticles (AgNPs)
The reduction of Ag+ was achieved by adding 5 ml of each Z. capense crude aqueous extract to 20 ml AgNO3 (1 mM) (Sigma Aldrich, South Africa). The solutions were incubated for 24 h in the light at room temperature. The change in colour from light yellow to dark brown was indicative of the presence of AgNPs [16, 52]. The procedure was repeated with the incubation for 30 min at 80 °C. All syntheses were performed in triplicate.
2.4. Quantification of AgNPs
Each AgNP solution was subjected to centrifugation using an Eppendorf centrifuge (model 5804/5804 R, USA). The six treatment solutions (leaves, knobs and roots at room temperature and at 80 °C) were separately transferred into pre-weighed Eppendorf tubes and purified for 2 h at 5000 rpm and at 4 °C. The supernatant from each solution was decanted, while the pellet was reconstituted in 20 ml sterile distilled water and the centrifugation step was repeated three times for effective removal of unreacted materials. The samples were then oven-dried at 50 °C for 24 h after which the tubes were re-weighed to obtain the yield of the synthesised AgNPs.
2.5. Morphological analysis of AgNPs and characterisation of the bioreductants
The bioreducibility of Ag1 to Ag0 was evaluated using a Shimadzu UV-2600 UV–vis spectrophotometer (Japan) at a range of 200–700 nm with a resolution of 1 nm. In order to evaluate the bioreducing and capping functional groups for the AgNPs, infrared spectra of the crude extracts and their corresponding biosynthesised AgNPs were obtained on a Perkin Elmer spectrum 100 Fourier transform infrared (FTIR) spectrophotometer (USA) with universal attenuated total reflectance (ATR) sampling accessory. The size and morphology of the AgNPs were examined using transmission and scanning electron microscopy (TEM and SEM, respectively). The TEM was also used to obtain selected area electron diffraction (SAED) of the AgNPs in order to evaluate their crystallinity. For TEM measurements, solutions of synthesised AgNPs were sonicated using a sonication bath (Soniclean, England) and the evenly dispersed AgNPs were coated onto carbon-coated TEM grids and placed under a lamp for the evaporation of the solvent before viewing. The SEM images were obtained on an ultra plus field emission gun scanning electron microscope (FEGSEM) (Carl Zeiss, Germany) operated at 5 kV accelerating voltage and equipped with energy dispersive x-ray analysis (EDX) (Aztec Analysis Software, England) to allow for the determination of the elemental composition. Dried samples were placed on aluminium stubs using carbon tape. The crude extracts were also subjected to 1H-NMR profiling. The NMR spectra were recorded using deuterated methanol (CD3OD) (Merck, Darmstadt, Germany) at room temperature on a Bruker AvanceIII 400 MHz spectrophotometer (Germany).
2.6. Surface sterilisation of Z. capense explants
Nodal explants of Z. capense from the nursery purchased and greenhouse maintained plants were cut to a length of about 12 ± 2 mm, washed 5 times under running tap water before being subjected to various surface sterilisation protocols. Explants were first rinsed in 70% ethanol for 1 min followed by a 5 min rinse in 3.5% sodium hypochlorite (NaOCl) and finally rinsed for 5 min in sterile distilled water three times. This procedure was repeated by replacing NaOCl with sodium dichloroisocyanurate (NaDCC). For NaDCC, explants were left in the dark (exposure times were 0.17, 0.5, 1.0, 6.0, 12.0 and 24.0 h) to reduce contamination. This was followed by washing once with sterile distilled water followed by a 1 min 70% ethanol rinse, then 3 times sterile distilled water rinse for 3 min each. In another sterilisation treatment, explants were treated with NaDCC as above for 6 h. They were further treated with the synthesised AgNP solution from the knobs at different concentrations (0, 25 and 50 mg l−1) for 30 min before culture. AgNPs synthesised from the knobs were selected because they represented samples of better morphology compared to the leaves and roots.
2.7. In vitro culture
Following surface sterilisation, all explants were edge trimmed and cultured on Murashige and Skoog basal salt medium (MS) [53] supplemented with 3% (w/v) sucrose, 2.0 mg l−1 benzylaminopurine (BAP), 1.0 mg l−1 indole-3-butyric acid (IBA) and AgNP solutions at concentrations of 0, 25, 50 or 100 mg l−1 in 20 × 100 mm2 culture tubes. All media were solidified with 1% (w/v) agar. The pH of the media was adjusted to 5.8 ± 0.2 before autoclaving for 20 min at 121 °C and 1.2 kg cm−2. All two-week-old sterile cultures, regardless of surface sterilisation treatment used, were removed and placed onto media containing BAP at 0, 0.5 and 1.0 mg l−1 singly or in combination with kinetin (KIN) at 0, 0.5, 1.0 and 2.0 mg l−1 following the procedure as described above.
2.8. Isolation and identification of fungal endophytes associated with Z. capense
2.8.1. Sample preparation and isolation of endophytic fungi.
Healthy leaf and stem cuttings of Z. capense were obtained from juvenile plants growing in the greenhouse or from a young tree at the School of Life Sciences, University of KwaZulu-Natal, Westville Campus. All samples were surface sterilised using an established method [54]. Potato dextrose agar (PDA) and rose bengal agar (RBA) (39 g in 1 l distilled water) were autoclaved at 121 °C for 15 min then cooled to 40–50 °C for 20 min. The agar media were prepared with or without antibiotic supplements i.e. chloramphenicol (10 µg ml−1), ampicillin (10 µg ml−1) or streptomycin (50 µg ml−1) and then poured into 9 cm Petri dishes. Thereafter, plates were inoculated with aseptically dried plant tissue segments (length 7.5 mm, 4 explants per plate), in triplicate for each sample type, incubated at 25 °C and observed daily for fungi growth.
2.8.2. Morphological and molecular identification of isolated fungi endophytes.
After 3 d in culture, plates with sufficient fungal growth were examined and fungal material transferred to fresh PDA plates to obtain pure cultures. Selection was based on differences in colony colour, size, growth pattern and consistency. For the extraction of DNA, mycelia were transferred from the PDA plates into potato dextrose broth in 250 ml Erlenmeyer flasks, incubated for 3 d at 28 °C, harvested and subjected to genomic DNA extraction using standard procedures [55] with the ZR fungal/bacterial DNA MiniPrep kit (Zymo Research, Irvine, California) following the manufacturer's instructions. The isolated DNA was diluted with tris-ethylenediaminetetraacetic acid (TE) buffer and stored at –20 °C for further use.
Polymerase chain reaction (PCR) was performed using the primers ITS4 (5'-TCCTCCGCTTATTGATATGC-3') and ITS5 (5'-GGAAGTAAAAGTCGTAACAAGG-3') [56]. The reaction was done in a 50 µl final volume containing 10 µl of genomic DNA, 2.5 µl of each primer (10 pM), 5 µl of Taq buffer, 0.25 µl Taq, 2 µl of MgCl2 (25 mM), 1 µl of dNTPs (10 mM) and made up with nuclease-free distilled water. The parameters employed for the PCR thermal cycle were: 95 °C for initial denaturation (2 min) and denaturation (30 s), annealing at 53 °C (45 s), extension at 72 °C (1 min) and final extension at 72 °C. The amplified PCR products were examined using gel electrophoresis (1.5% agarose gels in tris-acetate and TE buffer) and further purified using a GenElute PCR Clean-Up Kit® (Sigma, South Africa). Purified PCR products were sequenced by the Central Analytical Facilities DNA Sequencing service of the University of Stellenbosch (South Africa). Edited sequences were subjected to the Basic Local Alignment Search Tool Nucleotide (BLASTN) program and comparisons were made with data available from the GenBank® databases (National Centre for Biotechnology Information, U.S. National Library of Medicine, Bethesda, Maryland).
2.9. Statistical analyses
One-way analysis of variance (ANOVA) followed by Tukey's post-hoc test was used to determine if there were significant (p < 0.05) differences among the means of the data sets for nanoparticle yield and size and percentage contamination during in vitro culture. All statistical analyses were done using the statistical package for social sciences (SPSS, Version 23, IBM Corporation, Cornell, New York).
3. Results and discussion
3.1. Synthesis and quantification of AgNPs
The aqueous solutions of the biosynthesised AgNPs from leaves, knobs and roots had similar shades of dark brown indicative of AgNPs [16, 57] due to the reduction of Ag1 to Ag0 as reflected from the changes in electronic energy level [58]. The temperature-enhanced synthesis (80 °C) produced a more intensely coloured solution than the synthesis at room temperature. At elevated temperature, the molecules' kinetic energy increases which speeds up the consumption of silver ions thus reducing the chances for particle size growth [59]. This explains the overall increase in reaction rate and the smaller particles of uniform size distribution (figure 10). The leaves at 80 °C produced the highest yield (0.027 g AgNPs per g of plant (dry mass)) (figure 1). Plant pigments in addition to other secondary metabolites present in the leaves of Z. capense may have contributed to the increase in the yield of AgNPs. Recently, the pigments, pheophytin a and lutein were isolated from the leaves of Z. capense [60]. Lutein is a naturally occurring carotenoid, having a structure analogous to fucoxanthin which was previously reported to be responsible for the bioreduction of silver ions using Amphora sp. [61]. Leaves and roots produced higher yields of AgNPs compared to knobs (figure 1). This may be due to higher concentration of the phytochemicals in the leaves and roots.
Figure 1. Yield of AgNPs produced from leaves, knobs and roots of Z. capense at room temperature (RT) and at 80 °C (g AgNPs per g of plant material (dry mass)). Values represent mean ± SD (n = 3). Columns labelled with different letters (a)–(d) are significantly different at p < 0.05.
Download figure:
Standard image High-resolution image3.2. UV–visble spectral analysis
The formation of AgNPs was confirmed by the absorption peaks observed along the absorption band of the surface plasmon resonance (SPR) of the AgNPs free electrons [57]. Absorption peaks were observed at 440, 435 and 450 nm for the leaves, knobs and roots, respectively (for room temperature and temperature-enhanced synthesis, figure 2). The occurrence of the SPR band at about 440 nm and the broadness of the peaks are indicative of the spherical shape [62] and dispersion [63] of nanoparticles, respectively. The nanoparticles synthesised from knobs were the best dispersed as confirmed in both TEM and SEM images. Higher absorption peak intensities were observed for AgNPs synthesised from leaf extracts and knob extracts at 80 °C while AgNPs synthesised from root extracts at room temperature had a higher intensity. A progressive increase in the intensity of absorption peaks was also observed in previously reported temperature-enhanced AgNP biosynthesis [64, 65].
Figure 2. UV–visible spectra of AgNPs synthesised from Z. capense leaves (a), knobs (b) and roots (c) at room temperature and at 80 °C.
Download figure:
Standard image High-resolution image3.3. Fourier transform infrared spectroscopic analysis
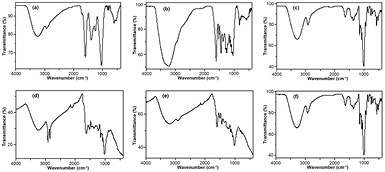
The Ag1 to Ag0 reducing potential of Z. capense can best be explained by the nature of the bioreducing phytocompounds in the extracts. The FTIR spectra of AgNPs synthesised using the leaves, knobs and roots of Z. capense and their corresponding crude extracts are presented in figure 3. The spectra show the vibrational frequencies of the various functional groups present in the crude extracts and those retained in the biosynthesised AgNPs. Absorption peaks from 3191 to 3272 cm−1 are characteristic of OH stretching in phenolics and sugars. Most the compounds (especially the benzophenanthridine alkaloids) that have previously been isolated from Z. capense have almost all their nitrogen atoms in either the tertiary or quarternary state and the few –NH containing compounds such as pellitorine and rutaecarpine are not soluble in water hence the lack of peaks for primary and secondary amino groups. The peaks around 2920 cm−1 are assigned to C–H stretching including those of methoxy compounds [66]. The peaks at 1620 cm−1 correspond to the alkenyl –C=C– stretching while those between 1400 cm−1 and 1515 cm−1 are for the C–C and C–N stretching, respectively. Bending vibrations of arenes, alkenes, amines and phenolics can be found in the region between 522 cm−1 and 933 cm−1. The phenolic, amino, carbonyl and other functional groups retained in the FTIR spectrum of the AgNPs are representative of the AgNP stabilisers.
Figure 3. FTIR spectra of the crude aqueous extract of Z. capense leaves (a), knobs (b) and roots (c) and that of the AgNPs synthesised (at 80 °C) from the leaves (d), knobs (e) and roots (f).
Download figure:
Standard image High-resolution image3.4. 1H-NMR fingerprinting
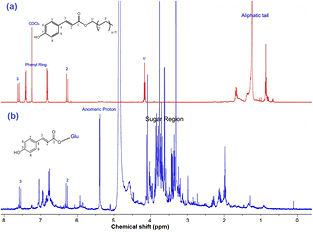
It has been widely reported that the bioactivity of most plant extracts is due to synergistic effects of the various active metabolites present in them [67, 68]. Studies on the green synthesis of AgNPs using plant material attribute the bioreduction of Ag1 to Ag0 to phytocompounds present in the plants [69, 70]. Since FTIR spectra only highlight classes of compounds based on their functional groups, 1H-NMR profiles were used to characterise extracts for the participating bioreducing molecules. NMR gives informative details regarding the chemical environment of the phytocompounds, thus regarded not only as the most relevant tool in the structural elucidation of organic compounds [71] but also a reliable technique for the qualitative and quantitative characterisation of metabolites in plant tissue extracts [72]. The 1H-NMR fingerprints of Z. capense's leaves, knobs and roots are presented in figures 4–6, respectively. The 1H-NMR spectrum of the aqueous extract of leaves showed prominent peaks in the sugar region with only two distinct peaks downfield having an ortho coupling constant (J = 8.6 Hz), suggesting a phenolic chemical environment. More aliphatic peaks were observed in leaves than knobs and roots. Dodecyl-trans-p-coumarate (figure 5, spectrum (a)) was previously isolated from the knobs [60]. Even though the spectrum of the aqueous extract of knobs revealed the presence of the coumarate backbone (figure 5, spectrum (b)), the dodecyl aliphatic tail is weak and insignificant relative to the integral values of the coumaric protons while that of the sugar moiety is strong. Based on the 1H-NMR spectrum, supported by the 13C APT, HMBC correlation and comparison with literature [73], we can propose that the compound, 6-O-p-coumaroyl-β-D-glucopyranoside was mostly involved in the biosynthesis of AgNPs from the knobs. This is a clear evidence of the modification of the chemistry around the pure active compound (dodecyl-trans-p-coumarate) that was previously isolated from the knobs.
Figure 4. 1H-NMR spectrum of the aqueous extract of Z. capense leaves in CD3OD.
Download figure:
Standard image High-resolution imageFigure 5. 1H-NMR spectra of dodecyl-trans-p-coumarate in CDCl3 (a) and aqueous extract of Z. capense knobs in CD3OD (b).
Download figure:
Standard image High-resolution imageFigure 6. 1H-NMR spectrum of the aqueous extract of Z. capense roots in CD3OD.
Download figure:
Standard image High-resolution imageFor the 1H-NMR spectrum of root extracts, more peaks were observed in the deshielded region that can be assigned to the aromatic protons of the benzophenanthridines. The aromatic protons of 6-hydroxydihdrochelerythrine, one of the compounds previously isolated in our laboratory fall between 7.1 and 7.7 ppm, whose methylenedioxo group and –NCH3 resonate at 6.0 and 2.7 ppm, respectively, while the 3.9 ppm resonance of the methoxy groups overlap with the sugar peaks. Benzophenanthridine alkaloids and lignans having –OCH2O– (methylenedioxo) have previously been reported from Z. capense [74]. The process of fractionation and repeated chromatography employed in the attempt to isolate pure phytoconstituents from plant extracts sometimes modify or in some cases changes the chemistry of the compounds while some compounds are lost [75] as observed in this study (dodecyl-trans-p-coumarate was isolated as pure compound during the phytochemical investigation of the knobs while the NMR profile of the unfractionated crude knob extract revealed strongly the presence of the glycoside of dodecyl-trans-p-coumarate).
The previous phytochemical studies on Z. capense showed differences in the compounds that make up the leaves, knobs and bark [30, 60, 74]. Moreover, the knobs, which received almost no attention in previous studies, have recently proven to be a potential source of naturally occurring anticancer compounds [60].
3.5. Scanning electron microscopy and EDX analysis
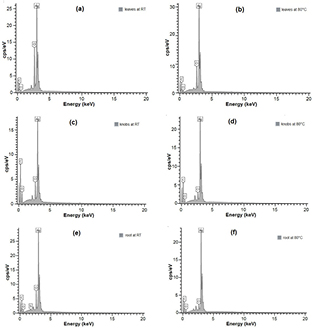
Figure 7 shows the SEM images of the synthesised AgNPs. Particles of similar size were well distributed and far less agglomerated in the knobs (figures 7(b) and (e)) compared to leaves and roots. The difference in degree of agglomeration may be attributed to slight differences in the structures of bioreducing compounds [76] or some physicochemical parameters like pH and concentration [77]. Flavonoids are known to be good stabilisers of AgNPs and so far, the only flavonoid isolated from Z. capense was obtained from the knobs [60]. This may have also contributed to the low agglomeration of the AgNPs biosynthesised using knobs. This was further substantiated with the EDX profiles (figure 8) with strong Ag peaks and weaker peaks for carbon, oxygen and other elements arising from the biomolecules that are surface-bound to the AgNPs. Silicon was observed only in the roots at room temperature (RT). At RT, percentage elemental silver in the knobs (48.4%) was significantly lower than leaves (69.3%) and roots (70.0%). Percentage elemental silver (CAg) increased with temperature in all cases (difference was only significant for the knobs), leaves (CAg were 69.3% at RT and 74.4% at 80 °C), knobs (CAg were 45.4% at RT and 66.9% at 80 °C), and roots (CAg were 70.0% at RT and 77.5% at 80 °C).
Figure 7. SEM images of AgNPs biosynthesised from the leaves, knobs and roots of Z. capense at room temperature (A–C, respectively) and at 80 °C (D–F, respectively).
Download figure:
Standard image High-resolution imageFigure 8. EDX spectra of AgNPs synthesised from Z. capense leaves (a) and (b); knobs (c) and (d); and roots (e) and (f) at room temperature (RT) and at 80 °C, respectively.
Download figure:
Standard image High-resolution imageThere seems to be a direct relationship between the AgNP yield and the percent elemental silver as knob-derived particles with the lowest yield had the least percentage of elemental silver, although there was no significant difference (data not shown) between the leaves' percentage elemental silver and that of the roots. Biosynthesis at elevated temperature was also observed to have increased the yield for both leaves and knobs but a decrease was noticed with the roots. These differences can best be explained by the nature of phytocompounds involved in the bioreduction and subsequent stabilisation of the AgNPs. In previous studies on green synthesis, temperature has been proven to have a strong influence on the yield and morphological characteristics of AgNPs [78–80]. However, AgNPs obtained from the knobs had the best morphological properties as revealed by the SEM and TEM images.
3.6. Transmission electron microscopy
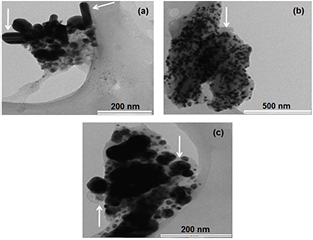
The shapes of the AgNPs as revealed by the TEM images were found to be predominantly spherical (figure 9) for the three extracts with a few nanorods seen from the leaf extract (figure 9(a)). The knobs gave the best nano-sized particle distribution ranging from 7 to 20 nm in the entire sample population while a wider range was observed in both leaves (4–28 nm) and roots (4–32 nm) (figure 10). Films of the biocapping molecules were observed around the AgNPs. This is important in that the former is responsible for the stabilisation of the AgNPs. Extracts from plants are known to contain phytocompounds serving dual purposes of both reducing Ag1 and stabilising Ag0 in the green-synthesis of size and shape-controlled AgNPs [81].
Figure 9. TEM images of AgNPs biosynthesised at 80 °C from the leaves (a), knobs (b) and roots (c) of Z. capense. Arrow heads point to nanorods (a) and films of biocapping molecules ((b) and (c)).
Download figure:
Standard image High-resolution imageFigure 10. Particle size (nm) distribution of AgNPs synthesised (at 80 °C) from the leaves, knobs and roots of Z. capense.
Download figure:
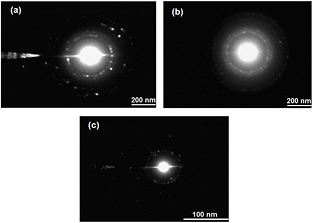
Standard image High-resolution imageDistinct rings were observed in the SAED patterns (figure 11) of the AgNPs. This confirms the polycrystalline nature of the biosynthesised AgNPs [82]. The appearance of rings made of more discrete spots are associated with larger crystallite (grain) sizes as observed in figures 11(a) and (c) while finer grain size produces more continuous rings of less discrete spots (figure 11(b)). This further confirms that the best nano-sized particles were from the knobs.
Figure 11. SAED patterns of AgNPs biosynthesised (at 80 °C) from the leaves (a), knobs (b) and roots (c) of Z. capense.
Download figure:
Standard image High-resolution imageMorphological characterisation of AgNPs was better achieved using the TEM than SEM. TEM clearly reveals the individual nanospheres and nanorods of the particles with their sizes as compared to the SEM which shows mainly the conglomerated environment of the AgNPs. The class, concentration and combining ratios of the numerous secondary metabolites in plants are known to vary across different plant organs [75] and this can be used to explain the observed differences in the characteristics of the AgNPs synthesised by each plant organ as observed in this study.
3.7. Surface sterilisation of Z. capense explants using NaOCl and NaDCC
The treatment using 70% ethanol with NaOCl did not show any potential for the sterilisation of Z. capense explants as all explants were contaminated with fungi after 3 d in culture (data not shown). Replacing NaOCl with sodiumdichloroisocyanurate (NaDCC) gave better results (table 1) probably due to the source of available chlorine. NaDCC is an organic form of chlorine having a solution pH of about 7, at which point hypochlorous acid (HOCl) makes up 80% of the solution's ionic constitution [83].
Table 1. Percentage Z. capense explants contaminated after various treatments with NaDCC after a 1-week culture period.
| NADCC treatment number | Treatments | % contaminated | |
|---|---|---|---|
| NaDCC (ppm) | Time (h) | ||
| N1 | 1000 | 0.17 | 100.0 ± 0.00a |
| N2 | 1000 | 0.5 | 98.7 ± 1.15a |
| N3 | 1000 | 1.0 | 96.7 ± 2.89a |
| N4 | 2000 | 0.17 | 80.0 ± 0.00b |
| N5 | 2000 | 0.5 | 78.3 ± 2.89b |
| N6 | 2000 | 1.0 | 60.7 ± 1.15c |
| N7 | 5000 | 6.0 | 40.0 ± 0.00d |
| N8 | 5000 | 12.0 | 15.0 ± 0.00e dns |
| N9 | 5000 | 24.0 | 1.7 ± 2.89f dns |
Note: All explants that did not survive one week of culture are denoted (dns). Percentages are given as mean ± SD. Means followed by the same superscript letter (a–f) are not significantly different using Tukey HSD test at 5% probability level (n = 3).
Explants from treatments N8 and N9 had the highest percentage of sterilisation. However, necrosis set in within one week of culture. Exposing Z. capense explants to NaDDC beyond 12 h is therefore not suitable since this resulted in very high necrosis even though explants displayed little to no contamination. As concentration of NaDCC increases with the exposure time, the level of fungal contamination decreases with subsequent increase in necrosis.
3.8. AgNP-mediated decontamination of Z. capense explants
Table 2 shows the potential of synthesised AgNPs in the control of in vitro contamination in Z. capense explants. Treatments T3, T5 and T10 had the best results with only 25% contamination. About half of the explants from T4, T6, T8 and T11, 27% from T7 and 25% of explants in treatments T9 were free of contamination. Nearly all explants were contaminated in T12 while all explants in the control (T1) (which had no AgNP treatment during both surface sterilisation or in the culture medium) were contaminated. Increasing concentration of AgNPs from 50 to 100 mg l−1 in the media (T3 and T4) showed a decline in the percentage of explants without contamination (75 to 50%, respectively). In the Ag rinse-only treatment, a good result (75% of explants not contaminated) was obtained at 25 mg l−1 concentration (T5).
Table 2. Percentage Z. capense explants contaminated after various treatments with AgNPs after a two-week culture period.
| Treatment | Treatments | % contaminated | |
|---|---|---|---|
| AgNPs rinse (mg l−1) | AgNPs in medium (mg l−1) | ||
| T1 | 0 | 0 | 100.0 ± 0.00a |
| T2 | 0 | 25 | 97.7 ± 2.52a |
| T3 | 0 | 50 | 25.0 ± 5.00d |
| T4 | 0 | 100 | 50.3 ± 2.08c |
| T5 | 25 | 0 | 25.0 ± 10.44d |
| T6 | 25 | 25 | 50.0 ± 0.00c |
| T7 | 25 | 50 | 73.3 ± 16.07b |
| T8 | 25 | 100 | 51.0 ± 3.61c |
| T9 | 50 | 0 | 75.0 ± 5.00b |
| T10 | 50 | 25 | 25 ± 3.00d |
| T11 | 50 | 50 | 51.0 ± 1.00c |
| T12 | 50 | 100 | 99.4 ± 0.81a |
Note: T is rinse treatment with AgNP solution with or without AgNP added to the culture medium. Percentages are given as mean ± SD. Means followed by the same superscript letter (a–d) are not significantly different using Tukey HSD test at 5% probability level (n = 3).
Higher concentrations of AgNPs in both the surface sterilisation stage and in the culture media were not effective in the control of contamination as observed in treatment T12 which had no uncontaminated explants after the culture period. It is possible that the high Ag concentration was lethal for the cell wall, thereby allowing for the release of endogenous fungi. More so, there was emergence of fungi after the two-week culture period suggesting that AgNPs did not completely inhibit fungi contamination in Z. capense but rather delayed its emergence.
All sterile cultures, regardless of sterilisation treatment, were used for shoot multiplication and the results are presented in table 3. The highest percentage of explants with shoots (88%) was recorded in explants cultured on 0.5 mg l−1 BAP while 1 mg l−1 KIN resulted in 74% explants producing shoots. Combining plant growth regulators (PGRs) for shoot multiplication led to a decrease in percentage of explants with shoots as observed in treatments 5–8.
Table 3. Effect of benzylaminopurine (BAP) and kinetin (KIN) on the development of shoots from the nodal explants of Z. capense for a culture period of 21 d.
| Treatment | Medium | % of explants with shoots | Number of shoots/explant (mean ± sd) | % fungal contamination | |
|---|---|---|---|---|---|
| BAP (mg l−1) | KIN (mg l−1) | ||||
| Control | 0 | 0 | 33.3 ± 8.30e | 2.42 ± 3.1a,b | 58.1 ± 0.35b |
| 1 | 0.5 | 0 | 88.6 ± 7.60a | 4.78 ± 3.3a | 22.2 ± 2.31f |
| 2 | 1.0 | 0 | 52.0 ± 5.29c | 1.50 ± 2.0b | 66.6 ± 0.51a |
| 3 | 0 | 1.0 | 74.3 ± 4.04a,b | 2.83 ± 2.8a,b | 41.6 ± 1.50d |
| 4 | 0 | 2.0 | 50.9 ± 1.62c | 1.58 ± 2.3a,b | 66.7 ± 0.06a |
| 5 | 0.5 | 0.5 | 66.9 ± 2.16b,d | 1.67 ± 2.0a,b | 41.8 ± 0.95d |
| 6 | 0.5 | 1.0 | NS | NS | 16.8 ± 1.11g |
| 7 | 1.0 | 0.5 | 33.5 ± 4.85e | 0.67 ± 1.2b | 50.0 ± 0.50c |
| 8 | 1.0 | 1.0 | 52.7 ± 6.42c,d | 1.17 ± 1.9b | 26.3 ± 1.53e |
Note: NS is no shoot. Percentages are given as mean ± SD. Means followed by the same superscript letter (a–g) within a column are not significantly different using Tukey HSD test at 5% probability level (n = 3).
The inhibitory effect of combined PGRs in this study shows that Z. capense may possess an appreciable concentration of some endogenous PGRs, thus limiting its requirement from an external source. The result obtained from the use of BAP in the present study agrees with those reported in the propagation of Zanthoxylym piperitum [51] and Zanthoxylym zanthoxyloides [41].
The present study shows that AgNPs controlled, to a large extent, the contaminants associated with Z. capense explants in in vitro culture, having a sterilisation potential greater than those of conventionally utilised sterilants, NaOCl and NaDCC. However, the synthesised nanoparticles could not achieve a complete removal of the fungal pathogens but rather delayed their emergence. This suggests that Z. capense may be a natural habitat for endophytic fungi which are mostly beneficial to their host plants in a symbiotic relationship [84]. A few members of the genus Zanthoxylum were reported to be hosts for certain endophytic fungi [85]. It is not fully understood whether the tissue culture of such plants allows the mutual benefit to continue or the relationship becomes lethal, but it has been reported that some fungi may not be pathogenic in vitro despite their prolificity in cultures [84]. This may explain why some shoots appear healthy even in fungal contaminated culture tubes. The role played by PGRs cannot be overemphasised in micropopagation studies as they control several physiological processes in plants either as biological stimulants or inhibitors [86]. The cytokinin, BAP has been used for induction or proliferation of shoots in several plants including two species of Zanthoxylum, Z. piperitum and Z. zanthoxyloides. PGRs are generally used in low concentrations either singly or combined with others. BAP was found to be more effective when used singly in this study. A similar result was obtained for the shoot proliferation experiment in Z. piperitum [51]. Nevertheless, a contrasting result was obtained in the micropropagation studies of Z. zanthoxyloides where a combination of BAP and IBA gave better results [41]. These also show that the PGR requirements vary from species to species within the same genus.
3.9. Z. capense's associated endophytic fungi
Morphological screening and the sequencing results of the ITS-18S rDNA led to the identification of 15 isolates of fungal endophytes from Z. capense. The fungal strains (accession number) included Alternaria sp. YLN10 (KC139496.1), Colletotrichum boninense (KX197406.1), Colletotrichum gloeosporioides (KP145439.1), Colletotrichum sp. X4 (KJ958362.1), Diaporthe actinidiae (KT163360.1), Diaporthe kongii (KR024740.1), Fusarium equiseti (HM008677.1), Guignardia mangiferae (EU677817.1), Neofusicoccum sp. GT4 (KC507279.1), Penicillium glabrum (KJ475813.1), Phomopsis sp. F89 (KM979832.1), Stagonosporopsis cucurbitacearum (KR085970.1), Xylaria sp. D14b2 (JQ341084.1), an uncultured Ascomycota clone ITS-11 AR (KJ461402.1) and an uncultured fungal clone ITS11 AR (EU718657.1). A total of 12 fungal species were identified in the field-derived stem explants while nine species were found in stem explants obtained from the green house. Overall, stems had a higher diversity of fungi compared to leaves where only six of the 15 isolates were found. Only 2 fungal strains (C. boninense and C. gloeosporioides) were present in both greenhouse and field samples indicating a preference of fungal strains for different environments. Environmental factors, differing across geographical locations, are known to have a significant influence on the abundance of fungal species in a specific plant [87].
4. Conclusion
In this study a bio-inspired synthesis of AgNPs using the leaves, knobs and roots of Z. capense is reported. The results show that biosynthesis at elevated temperature is needed to increase the yield of nanosilver from leaves and knobs only. The size of the particles and their corresponding elemental Ag content were different across the three plant parts. NMR spectra provided a better picture of the bioreducing phytocompounds compared to vibrational frequencies of the functional groups. Knobs, a rather ignored plant organ, can be employed for the biosynthesis of AgNPs with widely applicable morphological properties. A self-rescue approach to microbial contamination control was carried out as the leaves, knobs and roots of Z. capense were used for nanosilver biosynthesis which was in turn employed for decontamination of its explants used in preliminary tissue culture studies of this threatened species. Biosynthesised AgNPs could not prevent fungal contamination but rather delayed its emergence. However, identification of the endophytic fungi in Z. capense may lead to effective strategies for the control of in vitro fungal contamination in this species. BAP is a good candidate for shoot proliferation of Z. capense. This can be employed in further plant regeneration studies of this species by combination with varying ratios of low concentration auxins and/or other cytokinins.
Acknowledgments
We are grateful to Mr Philip Cristopher and Mr Vishal Bharuth of the Microscopy and Microanalysis Unit (MMU), University of KwaZulu-Natal, Westville, Durban. We also appreciate the National Research Foundation (NRF) and the UKZN Nanotechnology Platform for their financial support and the University of KwaZulu-Natal (UKZN) for providing research facilities for this work.