Abstract
Proteins suffer changes in their tertiary structure when they are immobilized, and enzymatic activity is affected due to the low biocompatibility of some supporting materials. In this work immobilization of lysozyme on carbon nanotubes previously functionalized by microwave irradiation was studied. The effectiveness of the microwave-assisted acid treatment of carbon nanotubes was evaluated by XPS, TEM, Raman and FTIR spectroscopy. The carboxylic modification of nanotube surfaces by this fast, simple and feasible method allowed the physical adsorption and covalent linking of active lysozyme onto the carbonaceous material. Thermal inactivation kinetics, thermodynamic parameters and storage stability were studied for adsorbed and covalent enzyme complexes. A major stability was found for lysozyme immobilized by the covalent method, the activation energy for inactivation of the enzyme was higher for the covalent method and it was stable after 50 d of storage at 4 °C. The current study highlights the effect of protein immobilization method on the biotechnological potential of nanostructured biocatalysts.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The use of enzymes in industry has been the focus of many investigations, because of its catalytic efficacy and stereoselectivity [1–3]. Temperature and solvent are main factors that hamper many applications. Therefore, enzyme immobilization procedures into solid materials are used to prevent denaturalization. Nanostructures from metal [4], ceramic [5] and polymer [6] sources have been used for this purpose, as well as carbon-based nanomaterials such as carbon nanoparticles, carbon nanotubes (CNT), among others. In particular, CNT are convenient due to their nanometric size and mechanical strength, besides their chemical and physical properties, which allow them to interact with biomolecules like proteins [7–11]. Moreover, the inherent properties of CNT provide a broad range of opportunities in biotechnological applications; i.e. biocatalysis [9], biofuel cell [12, 13], biosensors [14, 15] and drug delivery devices for cancer treatment [10, 16, 17].
A typical approach for conjugation of protein with CNT involves the chemical modification of nanotube sidewall, by incorporating functional groups such as carboxylic moieties. Common experimental procedures combine acid treatment with sonication [18], which may lead to collateral damage of the graphitic structure of CNT [19]. Microwave irradiation has also been used to a lesser extent as a fast, efficient and simple method to modify CNT, which results in a high degree of functionalization [20], particularly increasing carboxylic group attachment [21].
The enzyme immobilization onto nanomaterial surfaces may cause changes into the tertiary structure of protein, inhibiting its activity [8]. The interaction CNT-biomolecule has been determined by the immobilizing method: physical adsorption [22] or covalent binding [7]. The adsorption method (entrapment, adsorption, microencapsulation) involves physical forces; i.e. van der Waals, hydrophobic interactions, and hydrogen bonding; the process is reversible and it is recognized as a quick procedure to load large amounts of proteins on modified or non-modified CNT. On the other hand, chemical method (covalent attachment, cross-linking, ionic binding, conjugation by affinity ligand) encompasses covalent and ionic bonds by multiple step reactions which result in an irreversible process of enzyme immobilization [23].
Lysozyme (Lyz) is an enzyme used in the food industry due to its antibacterial activity [24]. Lyz activity has been studied at low temperatures, which is desirable in food processing where low temperature is critical to prevent bacterial growth [25]. The immobilization of Lyz onto CNT has been studied to understand the changes of the tertiary structure [22], as well as the rheological behavior of the CNT-Lyz dispersion [26] for possible applications. Most researches about CNT-Lyz complexes have mainly been focused on elucidation of the interaction between the protein and the graphitic material; for example, mechanism of the protein adsorption on functionalized CNT has been derived from adsorption isotherm assays [22, 27]. However, reports are rare on the thermodynamic analysis of the stability of Lyz-CNT complexes using experimental data from thermally induced denaturalization process.
In this work a quick and facile treatment was used to functionalize multiwalled carbon nanotubes (MWCNT) as precursors to form stable and biocatalytic complexes. Lyz was immobilized onto modified-MWCNT by adsorption and covalent binding. In a novel approach, kinetics and thermodynamic parameters for the thermal inactivation of Lyz bioconjugates were determined, as well as their storage stability, in order to evaluate the effect of the protein immobilization method on the biotechnological potential of this nanostructured biocatalyst.
2. Materials and methods
2.1. Materials
Lysozyme from chicken egg white (41 800 units mg−1, solid, 44 700 units mg−1 protein), multiwalled carbon nanotubes (MWCNT, 98% carbon basis, OD × ID × L = 10 nm ± 1 nm × 4.5 nm ± 0.5 nm × 3–6 µm), Micrococcus lysodeikticus ATCC No 4698 lyophilized, N-(3-dimethylaminopropyl)-N'-ethyl carbodiimide hydrochloride (EDAC) purum ⩾98.0% (AT), N-hydroxysuccinimide (NHS) ⩾98% and 2-(N-morpholino)ethanesulfonic acid (MES) ⩾99.5% were purchased from Sigma–Aldrich. Sulfuric acid (95–97%) was purchased from Merk and nitric acid (70%) was purchased from Faga Lab.
2.2. Surface modification of MWCNT
MWCNT were modified by microwave-assisted treatment in a strong acid mixture of concentrated sulfuric and nitric acids (3:1). The MWCNT containing acid suspension (1 mg ml−1 nanotube concentration) was placed in a microwave reactor (CEM Discover) at 150 W and 70 °C during 1 h. After treatment, the suspension was transferred to a cellulose acetate dialysis membrane (molecular mass cutoff about 12 kDa) and dialyzed against Milli-Q water, replacing the dialysis with fresh water until the pH remained steady. The black color suspension was frozen and lyophilized to obtain dry oxidized MWCNT (MWCNTox).
2.3. CNT derivatization with Lyz
2.3.1. Adsorption method (CNT-Lyz-Ads).
The adsorption experiments were carried out using a batch adsorption technique. A portion of MWCNTox (2 mg) was added to 10 ml of phosphates buffer (50 mM, pH 6.1) containing 1 mg ml−1 of protein and the resultant suspension was incubated with stirring at 4 °C overnight. Afterward, the suspension was centrifuged and washed in triplicate with MES buffer (50 mM, pH 6.1) to remove non-adsorbed protein. The pellet was resuspended in 5 ml of the same MES buffer and stored at 4 °C.
2.3.2. Covalent method (CNT-Lyz-Cov).
A portion of MWCNTox (2 mg) was suspended in 1.2 ml (10 mg ml−1) of EDAC solution to form an activated ester in the presence of 2.3 ml (50 mg ml−1) of NHS and 1 ml MES buffer (50 mM, pH 6.1). After stirring for 30 min at room temperature, the excess of EDAC and NHS was removed by dialysis against phosphate buffer (50 mM, pH 6.1). The activated ester groups were allowed to react with the amino moieties of proteins to form the amide bond by mixing the protein solution (1 mg ml−1) overnight at 4 °C. The MWCNT-protein complex was centrifuged and washed in triplicate with MES buffer (50 mM, pH 6.1) to remove free proteins. Finally, the MWCNT-protein complex was resuspended in 5 ml MES buffer and stored at 4 °C [7].
2.4. Characterization of MWCNTs and MWCNT-Lyz complexes
The MWCNTs were examined by x-ray photoelectron spectroscopy (XPS) on a Perkin Elmer PHI 5100 photoelectron spectrometer (USA) with Mg-Kα exciting irradiation, 10 kV, 300 W and 20 mA. The pressure in the analysis chamber was maintained approximately at 10−8 torr during each measurement. To compensate for surface charging effects, all binding energies were referred to C1s neutral carbon peak at 284.6 eV. The high resolution XPS spectra of C1s region was deconvoluted to determine the concentration of functional groups containing oxygen on CNT surface [28, 29].
Transmission electron microscopy was done using a JEOL 2010F microscope (Japan) operated at 200 keV and equipped with a XEDS spectrometer (Bruker). Sample suspensions were re-dispersed in deionized water through sonication and an adequate portion was transferred to copper grids for the analysis.
Fourier transform infrared (FTIR) spectra were recorded using a Perkin Elmer frontier spectrometer (England) by KBr pellet technique. The Raman spectra were recorded in Joriba LabRam spectrometer, using the exciting light of 488 nm Ar+ laser.
2.5. Enzyme activity assays
Activity assays for free and immobilized Lyz were carried out at 37 °C in phosphate buffer using M. lysodeikticus (pH 6.1, OD450 ≈ 0.7, 5 min, n = 3) as substrate. The bacteria lysis was detected by a decrease in the optical density at 450 nm (OD450).
For free Lyz (0.1 mg ml−1), 10 µl of Lyz solution were added to 1 ml of substrate M. lysodeikticus solution. Complexes (MWCNT-Lyz-Ads and MWCNT-Lyz-Cov) were used after preparation and 10 µl of complex solution were added to 1 ml of substrate (M. lysodeikticus) solution. The reaction was carried out in 1.5 ml cuvettes with 1 cm light path and the optical density was monitored after 5 min with lapses of 10 s with constant stirring using a CARY Varian 50 UV–vis spectrophotometer (Australia) equipped with thermostated cell. One unit (U) of Lyz produces an optical density (OD450) of 0.001 per minute in a pH 6.1 at 37 °C. For each assay blank correction was made, using the sample without enzyme [30].
2.6. Thermal inactivation assays
The thermal inactivation of free and immobilized Lyz complexes (adsorption and covalent) was studied by incubating the enzyme at different times without substrate in a range of temperatures between 30–60 °C in buffer MES (50 mM, pH 6.1). Free Lyz and MWCNT-Lyz complexes were cooled on ice and the enzymatic activity was measured immediately after incubation.
2.7. Kinetics and thermal parameters
The data obtained from the thermal inactivation kinetics were used to determine the thermal parameters using following equation [31, 32]
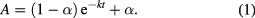
The inactivation rate constant  and the ratio of specific activities or remaining activity
and the ratio of specific activities or remaining activity  were calculated for every assay at different times
were calculated for every assay at different times  . The rate constant was determined for each temperature (T) and the activation energy
. The rate constant was determined for each temperature (T) and the activation energy  was estimated using the Arrhenius equation:
was estimated using the Arrhenius equation:

where  is the pre-exponential factor,
is the pre-exponential factor,  is the gas constant (8.314 J mol−1 K−1) and T is the absolute temperature (K). Half-life value (t1/2) of inactivation is given by the expression:
is the gas constant (8.314 J mol−1 K−1) and T is the absolute temperature (K). Half-life value (t1/2) of inactivation is given by the expression:

Enthalpy ( ) can be calculated once obtained the
) can be calculated once obtained the  , with equation:
, with equation:

Additionally, the standard free energy of Gibbs  for thermal inactivation was calculated with following equation [33]
for thermal inactivation was calculated with following equation [33]
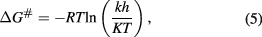
where  is the Planck constant (
is the Planck constant ( ) and
) and  is the Boltzmann constant (
is the Boltzmann constant ( .
.
Activation entropy  was calculated [34] by the expression:
was calculated [34] by the expression:
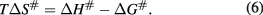
All calculations were done by nonlinear regression.
2.8. Stability to storage
Free and immobilized Lyz were stored at 4 °C for 50 d without substrate. The activity assay was carried out at different times with substrate M. lysodeikticus using conditions previously described (37 °C, OD450 ≈ 0.7, pH 6.1, 5 min).
3. Results and discussion
3.1. MWCNT functionalization and formation of MWCNT-enzyme complexes
MWCNTs are inherently hydrophobic, and they tend to agglomerate in aqueous solution. It is well known that functionalization of the MWCNT by oxidative treatment incorporates oxygen contained groups onto MWCNT surface, which promotes the hydrophilicity of nanotubes [10].
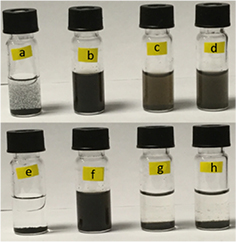
The effectiveness of functionalization treatment of CNT can be qualitatively evaluated by monitoring their suspension stability in aqueous media. Figures 1(a) and (b) show the appearance of as prepared pristine MWCNT and MWCNTox aqueous dispersion, respectively. Figures 1(e) and (f) depict the same dispersions of MWCNT and MWCNTox samples, respectively, after 24 h. As can be seen, pristine MWCNT precipitated, while MWCNTox remained in suspension as an indication of hydrophilicity after the oxidative acid treatment.
Figure 1. Images of as prepared (a) MWCNT pristine, (b) MWCNTox, (c) MWCNT-Lyz-Ads and (d) MWCNT-Lyz-Cov aqueous suspensions. In the same order, samples (e)–(h) are the suspensions after 24 h of preparation.
Download figure:
Standard image High-resolution imageFigures 1(c) and (d) include images of as prepared MWCNT-Lyz-Ads and MWCNT-Lyz-Cov aqueous dispersion, respectively, whereas figures 1(g) and (h) show the same dispersions of MWCNT-Lyz-Ads and MWCNT-Lyz-Cov, in that order, after 24 h. Both enzyme immobilization processes produced instability in the suspensions of the resulting complexes, which can be attributed to the weight increase of nanotubes due to Lyz attachment.
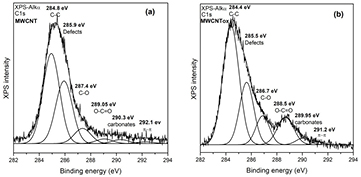
XPS analysis is a technique very effective to identify functional groups incorporated onto nanotubes by the microwave-assisted treatment. Figure 2 shows the deconvolution of high resolution XPS spectra of C1s signal for pristine MWCNT and MWCNTox samples. It can be noticed an increase of the contribution centered at 288.5 eV assigned to carboxylic groups, which changed from 2.57% in the original sample to 11.90% after microwave-assisted oxidative treatment. Li et al [35] modified single walled carbon nanotubes by ozone treatment and they determined by XPS an incorporation of 11.00% of carboxylic groups after 72 h of ozone treatment. Signals corresponding to C–C bonds (284.5 eV), π–π interaction (291.5 eV) and those of oxygen-contained groups such as C–O (287.4 eV) and carbonates (290.0 eV) were also identified in spectra of figure 2.
Figure 2. Spectral peak fitting of high-resolution C1s XPS signal of (a) MWCNT pristine and (b) MWCNTox samples.
Download figure:
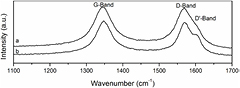
Standard image High-resolution imageRaman spectroscopy is a sensitive technique for characterization of CNT. The quantification of the intensity ratio between D and G bands (ID/IG) is a valuable tool to elucidate changes in the graphene sheet [36]. Curve (a) in figure 3 shows the spectrum of pristine MWCNT, in which it can be observed the G-band at 1568.7 cm−1 related to C–C stretching vibrations and the disorder induced D-band at 1346.5 cm−1 with an ID/IG of 1.02. Raman spectrum for MWCNTox (curve (b) in figure 3) displays the same peaks as a pristine sample; G-band at 1570 cm−1 and D-Band at 1348.3 cm−1 with an ID/IG of 1.04. Despite negligible change in the ID/IG ratio between pristine and oxidized MWCNT, there was detected an increase of the signal intensity at 1608 cm−1 corresponding to D'-band. This change was attributed to a double resonance feature induced by disorder and defects, as results of the high density of states for zone-edge and mid zone phonons [37]. This band has been observed by other researchers in surface modification of MWCNT [28, 37].
Figure 3. Raman G, D and D' bands of (a) MWCNT pristine and (b) MWCNTox samples.
Download figure:
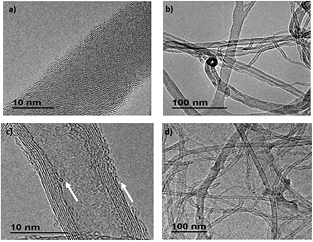
Standard image High-resolution imageMorphological changes on the surface of the MWCNT were analyzed by TEM. Figure 4 shows micrographs of pristine MWCNT ((a) and (b)) and MWCNTox ((c) and (d)) samples. The images revealed that the microwave-assisted treatment produced damage in outer and inner walls of nanotubes, as is pointed out in figure 4(c). There have been reported that acid treatments of nanotubes assisted by sonication often produce the fragmentation of nanotubes into shorter structures [18]. It is important to highlight that in this case, despite the partial damage observed in boundary walls of nanotubes, the nanotubes preserved their integrity without severe changes in their surface morphology and length (figure 4(d)).
Figure 4. TEM images of ((a) and (b)) MWCNT pristine and ((c) and (d)) MWCNTox samples.
Download figure:
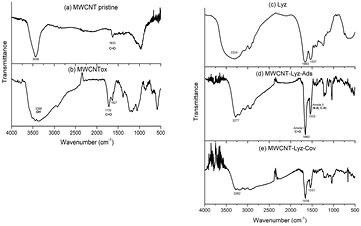
Standard image High-resolution imageFTIR technique was performed to corroborate the functionalization of MWCNT and subsequent enzyme attachment onto modified nanotubes. The spectrum of pristine MWCNT (figure 5(a)) shows bands at 3439 cm−1 and 1633 cm−1 attributed to the stretching vibration of O–H and C=O groups, respectively. This result was in agreement with XPS findings (figure 2(a)) and indicated that original nanotubes were partially oxidized. After the microwave-assisted acid treatment of MWCNT, a noticeable band appeared at 1725 cm−1 which were assigned to the carbonyl absorption of the carboxylic groups (figure 5(b)). Moreover, it was observed a broadening of hydroxyl band at 3388 cm−1 due to the increase of the contribution of carboxylic moieties. This clearly corroborates that carboxylic groups were successfully increased on nanotube surface by the treatment [9, 28, 29].
Figure 5. FTIR spectra of (a) MWCNT pristine, (b) MWCNTox, (c) free Lyz, (d) MWCNT-Lyz-Ads and (e) MWCNT-Lyz-Cov samples.
Download figure:
Standard image High-resolution imageThe spectrum of Lyz and Lyz attached by adsorption or covalent bond to CNT are also presented in figure 5. Figure 5(c) shows the spectrum of free Lyz, in which it can be observed a broad band centered at 3324 cm−1 assigned to OH group and peaks at 1663 cm−1 and 1542 cm−1 related to amide I (C=O stretching vibration) and amide II (mainly N−H bending vibration, coupled to C=O and C=C stretching), respectively. The spectrum of MWCNT-Lyz-Ads complex (figure 5(d)) was mostly similar to that of free Lyz, because the enzyme was coating the nanotubes [22]. MWCNT-Lyz-Cov (figure 5(e)) displayed spectral differences in the adsorption complex; it was observed a reduction of the absorption from 3400 to 3200 cm−1 due to the decrease of the O-H contribution of the carboxylic groups of the MWCNTox to form the amide bond with the enzyme. This is an indication of covalent attachment of Lyz to the surface of the MWCNTox. The peaks at 2930 cm−1 were assigned to the stretching vibrations of C−H bonds of the alkyl group [20].
It was found that the Lyz immobilized by adsorption and covalently linked onto MWCNTox preserved its activity against M. lysodeikticus. To achieve a difference between complexes, and taking in account the importance of immobilized enzymes for industrial applications, thermal and storage stability of the complexes were evaluated.
3.2. Thermostability
The stability of MWCNT-Lyz-Ads and MWCNT-Lyz-Cov at different temperatures was studied to elucidate differences between the methods of immobilization. Figure 6 illustrates the profile of thermal inactivation of the different complexes in the range of temperature of 30–55 °C. Experimental data of inactivation followed the first order monophasic kinetic model [38]. After 60 min of incubation, residual activity appeared to be constant for each assay. For the remaining activity, the MWCNT-Lyz-Ads sample (figure 6(a)) presented a major initial decrease and the  value (table 1) was higher in comparison with that of MWCNT-Lyz-Cov sample.
value (table 1) was higher in comparison with that of MWCNT-Lyz-Cov sample.
Table 1. Thermal and kinetics parameters of the inactivation of MWCNT-Lyz-Ads and MWCNT-Lyz-Cov complexes.
| MWCNT-Lyz |  (°C) (°C) |
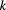 (h−1) (h−1) |
 |
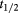 (h) (h) |
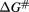 (kJ mol−1) (kJ mol−1) |
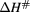 (kJ mol−1) (kJ mol−1) |
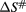 (J mol−1) (J mol−1) |
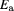 (kJ mol−1) (kJ mol−1) |
|---|---|---|---|---|---|---|---|---|
| Adsorption | 30 | 3.60 | 0.79 | 0.19 | 91.70 | 52.73 | −128.53 | 55.25 |
| 35 | 2.48 | 0.60 | 0.28 | 94.20 | 52.69 | −134.71 | ||
| 40 | 3.59 | 0.52 | 0.19 | 94.81 | 52.65 | −134.64 | ||
| 45 | 4.20 | 0.40 | 0.17 | 95.95 | 52.61 | −136.24 | ||
| 50 | 5.40 | 0.25 | 0.13 | 96.83 | 52.57 | −136.97 | ||
| 55 | 9.48 | 0.09 | 0.07 | 96.83 | 52.53 | −135.02 | ||
| Covalent | 30 | 1.20 | 0.80 | 0.58 | 94.47 | 69.16 | −83.47 | 71.68 |
| 35 | 2.36 | 0.64 | 0.29 | 94.29 | 69.12 | −81.68 | ||
| 40 | 2.73 | 0.53 | 0.25 | 95.44 | 69.08 | −84.19 | ||
| 45 | 4.36 | 0.33 | 0.16 | 95.73 | 69.04 | −83.89 | ||
| 50 | 6.71 | 0.25 | 0.10 | 96.07 | 69.00 | −83.80 | ||
| 55 | 10.42 | 0.14 | 0.07 | 96.36 | 68.95 | −83.51 | ||
Figure 6. Residual activity of thermal inactivation of (a) MWCNT-Lyz-Ads and (b) MWCNT-Lyz-Cov complexes.
Download figure:
Standard image High-resolution imageThe kinetic parameters of thermal deactivation (table 1) indicated that the complex by adsorption had an adverse impact in the stability against complex by covalent binding at low temperatures. The  values at low temperatures of MWCNT-Lyz-Ads were higher than those of MWCNT-Lyz-Cov, which mean that the inactivation is faster for adsorption complex. The activation energy was calculated for both complexes by the Arrhenius activation energy (Ea), being 55.25 kJ mol−1 (adsorption) and 71.68 kJ mol−1 (covalent), corroborating that is necessary more energy to deactivate the complex linked covalently.
values at low temperatures of MWCNT-Lyz-Ads were higher than those of MWCNT-Lyz-Cov, which mean that the inactivation is faster for adsorption complex. The activation energy was calculated for both complexes by the Arrhenius activation energy (Ea), being 55.25 kJ mol−1 (adsorption) and 71.68 kJ mol−1 (covalent), corroborating that is necessary more energy to deactivate the complex linked covalently.
The free energy of Gibbs (▵G#) of activation was positive for both complexes, and the inactivation was not spontaneous. The results showed a higher enthalpy (68.95 kJ mol−1) at a temperature of 55 °C for MWCNT-Lyz-Cov while the enthalpy for MWCNT-Lyz-Ads was found to be lesser (52.32 kJ mol−1). Higher enthalpy indicates an increase of the stability of the enzymatic system assuming that there are fewer bonds broken during inactivation [34]. However, the entropy of the systems was found to be negative for both complexes. Entropy change ( ) indicates the net enzyme and solvent disorder. Entropy is considered the most useful parameter in understanding the stability of proteins because when a protein molecule is deactivated, the disorder of the system increases which is direct measured of entropy [39]. Therefore, a negative entropy indicates that there is more stability in the deactivation of the complex linked covalently, associated with the ordering of enzyme—solvent system. After deactivation, water tends to form ordered clathrate structures around the non-polar molecule and this leads to a decrease of the entropy.
) indicates the net enzyme and solvent disorder. Entropy is considered the most useful parameter in understanding the stability of proteins because when a protein molecule is deactivated, the disorder of the system increases which is direct measured of entropy [39]. Therefore, a negative entropy indicates that there is more stability in the deactivation of the complex linked covalently, associated with the ordering of enzyme—solvent system. After deactivation, water tends to form ordered clathrate structures around the non-polar molecule and this leads to a decrease of the entropy.
3.3. Storage stability
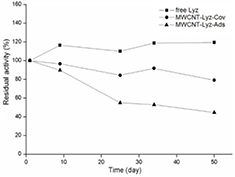
The storage stability of free and immobilized Lyz was studied by measuring the residual activity during 50 d of keeping at 4 °C (figure 7). It was notorious that the free Lyz had an increasing of the residual activity in the first 10 d after incubation. This behavior has been noted for other proteins [38, 40]; this is a fact that the enzymes may protect themselves against inactivation. On the other hand, MWCNT-Lyz-Cov and MWCNT-Lyz-Ads complexes showed a residual activity of 75 and 50%, respectively, after 50 d of storage. Based on these ultimate results and those of thermal stability, we state that the covalent link is more favorable for immobilization of Lyz onto CNT functionalized by microwave-assisted acid treatment.
Figure 7. Storage stability of free Lyz and complexes MWCNT-Lyz by adsorption and covalent binding.
Download figure:
Standard image High-resolution image4. Conclusion
MWCNT were efficiently modified by microwave-assisted acid treatment which allowed to attain 11.9% of carboxylic groups on nanotube surface. The treatment modified the morphology of boundary walls of nanotube without affecting neither the integrity nor their length of the individual unidimensional structures. The hydrophilicity of threated-MWCNT enabled to obtain stable aqueous suspensions without tendency to agglomerate by themselves. Active Lyz was successfully immobilized by adsorption and covalent binding onto functionalized-MWCNT. Free Lyz kept its catalytic activity for each assay, while the complex formed by adsorption and covalent binding presented a decreasing on the residual activity in the order: Free-Lyz < MWCNT-Lyz-Cov < MWCNT-Lyz-Ads.
The MWCNT-Lyz-Cov bioconjugate showed a higher stability at low temperatures (half-life value 0.58 h), and it was about three folds higher than that of MWCNT-Lyz-Ads (0.19 h). This behavior can be related with the nature of the interactions in the adsorption method, where predominant forces are van der Waals, hydrogen binding, electrostatic attraction, as well the π–π and the hydrophobic interactions. The comparison between both methods of Lyz immobilization onto CNT offered important information about the feasibility of the resulting bioconjugates, demonstrating that the use of covalent method increased the biotechnological potential of this biocatalyst material in industrial applications.
Acknowledgments
Financial support from Consejo Nacional de Ciencia y Tecnología (CONACYT) Mexico (Grant Ciencia Básica 2012-Nº 180280) is gratefully acknowledged. The authors thank Roberto Mora-Monroy from XPS Laboratory (Universidad de Sonora) and Eduardo Larios from TEM Laboratory (Universidad de Sonora). Daniel Puentes-Camacho acknowledges CONACYT for the scholarship during this study.