Abstract
In this study we report on the synthesis of silver nanoparticles (AgNPs) from the leaf extracts of Moringa oleifera using sunlight irradiation as primary source of energy, and its antimicrobial potential. Silver nanoparticle formation was confirmed by surface plasmon resonance at 450 nm and 440 nm, respectively for both fresh and freeze-dried leaf samples. Crystanality of AgNPs was confirmed by transmission electron microscopy, scanning electron microscopy with energy dispersive x-ray spectroscopy and Fourier transform infrared (FTIR) spectroscopy analysis. FTIR spectroscopic analysis suggested that flavones, terpenoids and polysaccharides predominate and are primarily responsible for the reduction and subsequent capping of AgNPs. X-ray diffraction analysis also demonstrated that the size range of AgNPs from both samples exhibited average diameters of 9 and 11 nm, respectively. Silver nanoparticles showed antimicrobial activity on both bacterial and fungal strains. The biosynthesised nanoparticle preparations from M. oleifera leaf extracts exhibit potential for application as broad-spectrum antimicrobial agents.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is a science centered on atomic, molecular and supramolecular molecules aiming to create nano-structures with enhanced functionalities [1], and the term nanoparticle describes particulate matter ranging in size from 1–100 nm [2]. Bearing a nano scale size offers the advantage of having a significantly large surface area to volume ratio [3]. Increased surface area, in combination with nanoparticle conformation and distribution in solution contribute to their enhanced physical and chemical properties which are useful in a variety of fields such as antimicrobial development [4], bio-molecular detection, diagnostics [5], catalysis [6], micro-electronics [7], sensing devices and targeting of drugs to cancer cells [8]. These wide ranging applications and the increasing ability to manipulate nanoparticle form and function has sparked great interest in the scientific community over the past decade, particularly in terms of the development of efficient and environmentally friendly synthetic methods.
Conventional physical and chemical methods presently have limited use in preparing metal nanoparticles due to toxic chemicals [9]. Moreover, these methods are associated with high-energy input and costly downstream processing [10].
Green synthesis is defined as the use of environmentally compatible materials such as bacteria, fungi and plants in the synthesis of nanoparticles [11]. These attractive green strategies are free of the short falls associated with conventional synthetic strategies, i.e. they are eco-friendly [12]. Alternatively, synthesis from biologically derived extracts offers several advantages such as rapid synthesis, high yields and importantly, the lack of costly downstream processing required to produce the particles [13–15]. Hence, nanoparticle synthesis from plant extracts tentatively offers a route for large scale production of commercially attractive nanoparticles.
Numerous studies report on the use of plant extracts to synthesise AgNPs with significant antimicrobial activities: leaf extracts of Acalypha indica [16], Solidago altissima [17], Xanthium strumerium L [18], Murraya koenigii (curry leaf) [19], Ocimum sanctum (Tulsi leaf) [20, 21], seed extracts of Acacia farnesiana [22], Macrotyloma uniflorum [23], root extracts of Trianthema decandra [24], stem extracts of Ocimum sanctum [20], and even fruit extracts of Musa paradisiaca (banana) peels [25] and Carica papaya [26]. In these studies phytocompounds in the plant extract serve as reducing and/or capping agents in the reaction with silver nitrate (AgNO3), a commonly used precursor in silver nanoparticle synthesis.
Moringa oleifera Lam (drumstick tree) is a tree species indigenous to north-western India but is also regarded as an important crop in several other countries such as the Philippines, Sudan, Ethiopia and South Africa [27, 28]. It belongs to the genus Moringa and the family Moringaceae [29], and is highly sought after for its tender pods, flowers and leaves, all of which are safe for human consumption [30]. The leaves, in particular, are recognised for their natural healing properties and are popularly consumed in a variety of ways [29, 31]. Research to date has revealed that extracts prepared from the leaves possess high natural antioxidant properties and some antibacterial activity against gram-positive and gram-negative bacteria [27, 32].
In the present study we report the biosynthesis of AgNPs from M. oleifera leaf extract using sunlight as the primary source of energy for nanoparticle formation from freeze-dried (FD) and fresh (F) leaf material. There are few published comparisons on nanoparticle yield, quality and bioactivity for extracts prepared with dry and fresh tissue within individual species. Moringa leaves represent a promising candidate for green synthesis of bioactive AgNPs that can be produced in an environmentally friendly manner.
2. Materials and methods
2.1. Materials
All chemicals, solvents and media used in this study were of analytical grade and purchased from Merck (Pty) Ltd, South Africa, unless stated otherwise. Antibiotics were purchased from Sigma Aldrich, Germany.
2.2. Preparation of leaf extract
Fresh M. oleifera plant material was harvested from the south coast of KwaZulu-Natal. Leaf material was separated from the stems, washed with distilled water and air dried to remove residual debris. A portion of the leaf material was placed in a freeze dryer (Edwards Wirsam, England) for 72 h until all moisture was removed. Thereafter, it was stored at −16 °C for further use. Methods used for the preparation of leaf extract samples and biosynthesis of AgNPs were adapted from Veerasamy et al [12]. Extracts were prepared by using 10 g of FD leaf material and the equivalent amount of F leaf tissue (in terms of dry weight), in replicates of four. Each replicate was homogenised thoroughly in 50 ml Millipore water (using a IKA T25 digital Ultra-Turrax-T 25 D, Germany) and the final volume was adjusted to 100 ml. Resulting homogenates were transferred to 250 ml Erlenmeyer flasks. Flasks were covered with foil and placed on a mechanical shaker (Labcon, Spo-MP8) at 115 rpm, for 24 h at room temperature. To obtain aqueous extracts, homogenates were subjected to vacuum filtration using Whatman no.1 filter paper (Whatman Limited, England).
2.3. Synthesis of AgNPs
An aliquot (5 ml) of aqueous plant extract sample was added to 50 ml of 1 mM aqueous AgNO3. To drive nanoparticle formation the reaction mixtures were exposed to direct sunlight. Colour change of the reaction mixtures were monitored to determine nanoparticle formation which is indicated by a dark brown colour. Once colour intensities of the solutions reached a maximum, the vessels were removed from sunlight and stored in darkness at room temperature to prevent agglomeration of the nanoparticles. A 50 ml aliquot of AgNO3 containing 5 ml distilled water was processed as described above and used as a negative control. Further confirmation of silver nanoparticle formation by the reduction of Ag+ from AgNO3 was achieved by UV–vis spectral analysis. Nanoparticle solutions were diluted 1:2 with distilled water and distilled water served as a blank. Nanoparticle solutions and the control were simultaneously scanned from 190–900 nm using a UV–vis spectrometer (Specord 210 Analytikjena, Germany).
2.4. Purification and concentration of AgNPs
The purification and concentration of AgNPs from the final reaction mixture was adopted from [33]. The 55 ml reaction mixture (50 ml AgNO3 + 5 ml leaf extract sample) (n = 4) was split into two equal parts and transferred to pre-weighed sterile 50 ml centrifuge tubes (United Scientific, South Africa). The preparations were then centrifuged at 4000 rpm for 2 h (Eppendorf centrifuge 5810 R, Germany), at 4 °C. Supernatants were discarded and the pellet was washed in 10 ml of distilled water to remove any contaminating plant material before centrifugation for 1 h. This wash step was repeated twice to remove water soluble biomolecules such as proteins and cellular metabolites. One half of each replicate was then dried in an oven at 37 °C for 24 h to determine the dry mass of the AgNPs (difference between mass of tube with nanoparticles, and mass of tube), whilst the other portion of each replicate was reconstituted in 1 ml of distilled water. The mass of each dried pellet was applied as the equivalent mass of its corresponding reconstituted pellet since each replicate was equally split. Thus, the concentration of AgNPs was determined on a mg ml−1 basis. This procedure was also used to determine the dry mass of the leaf tissue samples from 5 ml aliquots of the extracts. A comparison of nanoparticle yield between F and FD starting material was made using the mass of the AgNPs attained on a mg of silver nanoparticle per 1 g dry leaf tissue mass. Dry silver nanoparticle samples were kept at room temperature whilst reconstituted samples were stored at 4 °C prior to use.
2.5. Characterisation and analysis of AgNPs
Characterisation techniques were adopted from Gannimani et al [13]. Dry nanoparticle preparations were used for scanning electron microscopy (SEM, FEGSEM Zeiss Ultraplus), energy-dispersive x-ray (EDX) analysis at 20 kV to determine elemental composition of the particles, and Fourier transform infrared (FTIR, ALPHA-BRUKER, Germany) spectroscopy analysis, whereas reconstituted samples were used in transmission electron microscopy (TEM). Sample images were obtained using a JEOL TEM (1010) at an accelerating voltage of 100 kV. The sizes of the nanoparticles and data analysis of the sizes was accomplished using ITEM (soft imaging system, Germany ver 5.0).
2.6. Antimicrobial assays
2.6.1. Antibacterial assay.
Varying concentrations of the biosynthesised AgNPs were tested for bioactivity against Staphylococcus aureus (43300), Enterococcus faecalis (5129), Escherichia coli (35218), Pseudomonas aeruginosa (27853) and Klebsiella pneumoniae (700603). The broth microdilution method [34] was employed to determine minimum inhibitory concentration (MIC). Serial two fold concentrations of AgNPs and leaf tissue were prepared in sterile 96-well plates over the range of 200–1.25 μg ml−1. The first row (row A) of the plates were reserved for blank/negative and uninhibited growth controls and these wells were filled with Mueller Hinton (MH) media only. Wells were then inoculated with diluted overnight broth culture initially adjusted to 0.5 McFarland turbidity standards and incubated at 37 °C for 16–20 h. Subsequently, 40 μl of freshly prepared iodonitrotetrazolium chloride (INT) (or 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazo- lium chloride) solution (200 µg ml−1) was added to all wells and the plates incubated in the dark for 4 h at 37 °C. The INT reagent, initially colourless, was reduced to a red product following incubation. This reduction was the result of persistent bacterial growth, whilst no colour change denoted the inhibition or lack of bacterial growth. The absorbance was determined at 630 nm using a multimodal plate reader (Biotek Synergy HT, USA) with Gen 5 software (Biotek Synergy HT, USA Ver 2.01.14). Neomycin served as a positive control. The MIC was defined as the lowest concentration of an antimicrobial agent that inhibits the growth of a microorganism after overnight incubation.
2.6.2. Antifungal assay.
The same concentration range was used to determine the antifungal activity of AgNPs and leaf material according to the broth microdilution method as prescribed by the NCCLS guidelines [35]. Microtitre plates were prepared as described in the antibacterial assay with the exception that sabouraud-2% dextrose (SD) broth was used instead of MH broth. Three Candida reference strains, viz. Candida albicans (ATCC 90028), Candida krusei (ATCC 6258) and Candida parapsilosis (ATCC 22019) were inoculated into freshly prepared SD broth and grown aerobically overnight at 30 °C in an Infors HT Multitron environmental shaker (United Scientific, South Africa) at 150 rpm. Cells were harvested by centrifugation and thereafter re-suspended in 1% saline. The absorbance of resuspended starter cultures was determined spectrophotometrically at 600 nm. To conform to McFarland's standards, the cells were diluted using SD broth to achieve optical densities in the range of 0.08–0.10. Once achieved, the working suspension was diluted (1:20) in RPMI 1640 medium (with L-glutamine, without bicarbonate and phenol red, Biochrom, Berlin). The working suspension was further diluted (1:50) with RPMI 1640 to obtain the final test inoculum concentration of 1–5 × 103 CFU ml−1. Aliquots (100 μl) of inoculum were added to all wells of the test microtitre plates except the blank/negative control wells. The plates were incubated aerobically at 30 °C for 16–20 h. After incubation, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H terazolium salt (MTS, Promega Corporation, Madison, USA) was added to all wells and incubated in the dark at 30 °C for 4 h. Thereafter the absorbance was determined at 490 nm. The commercially available antibiotic Amphotericin B, was used as a positive control. All experiments were performed in triplicate.
2.7. Statistical analysis
All data were statistically analysed using SPSS version 21. All percentage data obtained from the biological assays were arcsine transformed and analysed for normality (p > 0.05) using a Kolmogorov–Smirnov test. Parametric data (which in some cases was the consequence of transformation) were subjected to an analysis of variance (ANOVA). Differences were considered significant at the 0.05 level and where possible, means were separated by a Tukey post-hoc test. Nanoparticle yield and size were analysed for normality as above and subjected to an Independent sample t-test.
3. Results
3.1. Colour change and UV–vis spectroscopy
A primary indication of silver nanoparticle formation is represented by a reaction solution colour change to dark brown [36]. Addition of AgNO3 to FD and F M. oleifera leaf extract samples produced an instantaneous colour change from an initial, yellow solution for both FD and F samples (figures 1(a) and (b)), to dark brown solutions (figures 1(c) and (d)) within 1 h of reaction time.
Figure 1. Colour change of reaction solutions containing FD ((a) and (c)) and F ((b) and (d)) leaf extract samples with AgNO3 at 0 h and 1 h.
Download figure:
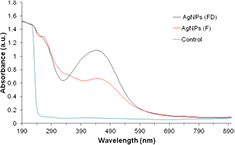
Standard image High-resolution imageThe surface plasmon resonance of AgNPs showed a peak centered near 440 nm and 450 nm respectively for both leaf extracts which corresponds to the absorbance of AgNPs (figure 2) [37].
Figure 2. UV–vis spectral profiles of silver nanoparticle containing solutions synthesised from FD and F M. oleifera leaf extract samples and control solution after 1 h of reaction.
Download figure:
Standard image High-resolution image3.2. Yield analysis
Nanoparticle yield for AgNPs prepared from FD leaf samples (0.91 ± 0.4 mg of AgNPs per 1 g leaf tissue dry mass) was statistically comparable (p > 0.05) to that obtained for AgNPs prepared from F samples (0.81 ± 0.2 mg AgNPs per 1 g leaf tissue dry mass) (figure 3).
Figure 3. AgNP yield from FD and F M. oleifera leaf extract samples (AgNPs/leaf tissue dry mass of mg g−1). Values represent mean ± SD (n = 4). p > 0.05 when yield was compared between FD and F leaf extract samples (t-test).
Download figure:
Standard image High-resolution image3.3. SEM and EDX analysis
Figures 4(a) and (c) represent SEM analysis of purified nanoparticle preparations. Furthermore, the particles were observed to be highly agglomerated, possibly an artefact of the centrifugation and subsequent drying required to prepare silver nanoparticle samples for SEM analysis. Silver nanocrystals typically display an absorption peak around 3 keV due to their surface plasmon resonance [36]. EDX analysis of AgNPs demonstrated a strongly well defined silver signal at 3 keV along with weak carbon, oxygen and nitrogen peaks, with the latter weaker signals probably representing surface biomolecule capping structures originating from the leaf extracts (figures 4(b) and (d)).
Figure 4. SEM images and EDX spectra of AgNPs prepared from FD ((a) and (b)), and F ((c) and (d)) leaf extract samples.
Download figure:
Standard image High-resolution image3.4. TEM analysis
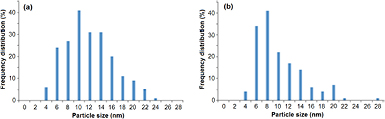
TEM analysis clearly illustrated that the particles are spherical in shape and appear well dispersed (figures 5(a) and (b)). Additionally, TEM analysis revealed the particles to possess narrow size distribution ranges with average sizes of 11 ± 4.3 nm and 9 ± 4.2 nm for FD and F AgNPs, respectively (figures 6(a) and (b)).
Figure 5. TEM images of AgNPs produced from (a) FD and (b) F leaf samples.
Download figure:
Standard image High-resolution imageFigure 6. Size distribution of AgNPs synthesised from (a) FD and (b) F leaf samples of M. oleifera. (n = 100, p > 0.05 when AgNPs prepared from F and FD samples were compared, t-test).
Download figure:
Standard image High-resolution image3.5. FTIR studies
The FTIR spectra of FD and F leaf material and AgNPs are shown in figure 7. The spectra revealed that similar or identical functional groups in both leaf sample types were responsible for binding and reducing Ag+. Functional groups corresponding to absorbance peaks in the range of 3000–3300, 2800–3000, 1626, 1400–1550, 1380–1403 and 1000–1100 cm−1 were observed. These peaks are known to be associated with stretching vibrations of hydroxyl groups in alcohols or phenolic compounds, CH2 and CH3 functional groups; C=C groups of aromatic compounds or C=O groups of carboxylic acids, amide I (CONH2) and amide II (CONH) groups, geminal methyls, ether linkages and C–O or C–O–C functional groups, respectively [38–40]. In contrast, these peaks were either absent or displayed drastically reduced intensities in the corresponding silver nanoparticle spectra. This seems to suggest that functional groups in this region are involved in the reduction and stabilisation of the AgNPs by their subsequent oxidation. Additionally, ether linkages, C–O and C–O–C functional groups are thought to be groups of flavones, terpenoids and polysaccharides present in the leaf biomass. These functional groups have been observed on the surface of AgNPs produced from leaf extracts of medicinal plants [41].
Figure 7. (a) FTIR spectra of FD leaf sample (plant) and AgNPs derived from FD leaf sample; (b) FTIR spectra of F leaf sample (plant) and AgNPs derived from F leaf sample.
Download figure:
Standard image High-resolution image3.6. Antimicrobial activity
3.6.1. Antibacterial studies.
The AgNPs synthesised from both leaf sample types displayed inhibition of gram-negative and gram-positive bacteria (table 1). Solutions of AgNPs from both leaf preparations at a concentration of 25 μg ml−1 inhibited the growth of K. pneumoniae, P. aeruginosa and S. aureus strains. Interestingly, a two-fold decrease in concentration (12.5 μg ml−1) of both nanoparticle preparations was observed to inhibit the growth of E. coli and E. faecalis. Both Moringa leaf extract samples did not display inhibitory activities throughout the concentration range evaluated in this study (data not shown). The commercially available antibiotic neomycin displayed MIC's ranging from 6.25 to 25 μg ml−1. The results of our study showed that AgNPs prepared in this study are not only more effective at lower concentrations but also display a broader susceptible bacterial spectrum range. It is noteworthy that E. faecalis, P. aeruginosa and S. aureus strains were insensitive to neomycin but susceptible to the AgNPs prepared from both leaf extracts.
Table 1. MIC of AgNPs synthesised from M. oleifera leaf extract samples, and a commercial antibiotic against bacterial strains.
| Organism | MIC (μg ml−1) | ||
|---|---|---|---|
| AgNPs synthesised from: | Neomycin | ||
| FD tissue | F tissue | ||
| E. coli | 12.5 ± 0.01 | 12.5 ± 0.01 | 6.25 ± 0.04 |
| E. faecalis | 12.5 ± 0.01 | 12.5 ± 0.00 | NI |
| K. pneumoniae | 25 ± 0.01 | 25 ± 0.02 | 25 ± 0.04 |
| P. aeruginosa | 25 ± 0.01 | 25 ± 0.01 | Ni |
| S. aureus | 25 ± 0.03 | 25 ± 0.04 | NI |
aCommercial antibiotic showed no inhibition in terms of effecting 80% kill at the evaluated concentrations. Results are expressed as mean ± SD. (p > 0.05 for FD and F AgNP preparations within each microorganism tested, ANOVA) NI is no inhibition.
3.6.2. Antifungal studies.
Three Candida reference strains were employed to evaluate the antifungal potency of the silver nanoparticle preparations (table 2). A concentration of 6.25 μg ml−1 AgNPs inhibited the growth of all fungal strains whilst the leaf extract samples did not display inhibition (data not shown). The pharmaceutical bioactive amphotericin B displayed a MIC of 0.02 μg ml−1 across all evaluated fungal strains.
Table 2. MIC of AgNPs synthesised from M. oleifera leaf extract samples, and a commercial antifungal preparation against fungal strains.
| Organism | MIC (μg ml−1) | ||
|---|---|---|---|
| AgNPs synthesised from: | Amphotericin B | ||
| FD tissue | F tissue | ||
| C. albicans | 6.25 ± 0.03 | 6.25 ± 0.02 | 0.02 ± 0.00 |
| C. krusei | 6.25 ± 0.02 | 6.25 ± 0.02 | 0.02 ± 0.00 |
| C. parapsilosis | 6.25 ± 0.02 | 6.25 ± 0.01 | 0.02 ± 0.00 |
Note: Results are expressed as mean ± SD (p > 0.05, for FD and F AgNP preparations within each microorganism tested, ANOVA).
4. Discussion
Given the wide ranging applications of AgNPs in recent years, many researchers have focused on the development of modified or novel synthetic strategies for AgNPs as opposed to the use of conventional methods which are strongly associated with toxic environmental footprints [11]. This study reports on the antimicrobial activities of AgNPs prepared from leaf extract samples of the medicinal tree species, M. oleifera by a green, cost effective synthetic method.
Up until now, green synthesis of AgNPs from leaf extract samples at room temperature have been shown to generally yield AgNPs at relatively slow rates. For instance, complete bioreduction of Ag+ from Aloe vera and Iresine herbstii leaf extracts occurred at 24 h and 168 h, respectively [33, 42]. Prasad and Elumalai reported rapid synthesis (10 min) of AgNPs from M. oleifera leaf powder at a reaction temperature range of 60 °C–80 °C [43]. As suggested by previous studies sunlight irradiation, a freely available bio-energy resource, was used to drive nanoparticle formation with desirable reduction times (5–15 min) [44–46]. Reduction of Ag+ by M. oleifera leaf extracts occurred instantaneously in the presence of direct sunlight and complete reduction was observed after 1 h. This was indicated by colour change of the reaction solutions to dark brown [47]. It has previously been reported that AgNPs display a brown colour in aqueous solution due to the surface plasmon phenomenon [12, 48]. The efficiency of the protocol used in the present study is evidenced by the fact that complete reduction occurred after 24 h at 50 °C, in the reaction of AgNO3 and carbohydrates secreted by Chorella vulgaris [39]. This seems to suggest that the time required for nanoparticle formation varies according to the nature of reducing agent. However, considering the reduction times reported for the use of sunlight irradiation as a primary energy source, this technology constitutes a promising alternative to the use of non-renewable energy sources for silver nanoparticle production.
Yield analysis indicated no significant differences between AgNPs prepared from FD or F leaf sample types. Although, it has previously been reported that the dehydration of plant material increases the availability of phytocompounds and would therefore increase the bioactivity of the plant material (in terms of their reducing ability) [49]. To date, silver nanoparticle yield from leaf samples is relatively unreported. However, silver nanoparticle yield produced in this study are comparable to previously derived AgNPs prepared from various organ extracts of Amaranthus dubius [50].
Microscopic analysis (SEM) provided an inconclusive indication that AgNPs were spherically shaped. In addition, the particles appeared to be highly agglomerated, possibly owing to the physical dehydration exerted during the SEM sample preparation procedure [50, 51]. In contrast, TEM analysis of aqueous silver nanoparticle samples provided unequivocal evidence that the prepared AgNPs were spherical in shape. Furthermore, the particles were observed to be stable and dispersed, even within aggregates. This finding is in accordance with previous reports for AgNPs derived from M. oleifera leaf extracts [43, 52], suggesting that the leaf extract of M. oleifera presents a dual functionality of reducing Ag+ and subsequently stabilising nascent AgNPs. Interestingly, a previous study on the influence of nanoparticle shape on bioactivity suggested that spherically shaped AgNPs display superior antimicrobial activities when compared to rod shaped AgNPs [53].
In recent years, however, studies have suggested that the bioactivity of AgNPs occur in a size-dependent manner with smaller particles exerting better bioactivities than larger ones [54, 55]. Size class distribution studies of the AgNPs prepared in this study indicate that the particles had narrow size distribution ranges and no significant differences were noted for the nanoparticle preparations in terms of their size. The particles produced here are relatively small. AgNPs synthesised from the leaf extracts of Ziziphora tenuior and Mimusops elengi at room temperature for example were found to be 20–83 nm in size [51, 56]. The introduction of heat in nanoparticle synthesis has been reported to significantly decrease the size of synthesised AgNPs [12]. Biosynthesised AgNPs derived from Cordia dichotoma leaf extract in a heat driven reaction (60 °C) were shown to be 10 nm in size using TEM [40]. However, AgNPs synthesised from M. oleifera leaf powder extract in a heat driven process produced particles with an average diameter of 57 nm [43]. It can be suggested that incubation at room temperature or heat strongly influences the size of generated nanoparticles according to the nature of the reducing agent. The use of sunlight in silver nanoparticle synthesis from Allium sativum and Andrachnea chordifolia extracts resulted in reported sizes of 7.3 nm and 3.4 nm, respectively. These findings and the data reported in this study strongly favour the use of sunlight for the production of AgNPs with small sizes.
EDX analysis revealed that in addition to silver, oxygen, carbon and nitrogen were also present on the nanoparticle surface. Similar results were reported previously [37, 57] and believed to originate from biomolecule capping structures [58]. Importantly, AgNPs with oxidised surfaces have been reported to induce the formation of 'holes' on the surface of bacteria [59], which suggests that the presence of oxygen on the surface of the nanoparticles prepared here may enhance their bactericidal activities.
FTIR analysis suggest the presence of phenolic compounds, flavones, terpenoids and polysaccharides in the leaf biomass as potential Ag+ capping agents. Interestingly, M. oleifera leaves are known to be a rich source of the antioxidant phenolic compounds: quercetin and kaempferol [27]. In particular, quercetin belonging to the flavonoid group of phenolic compounds has previously been shown to possess strong chelating ability [60]. This suggests that the reduction of Ag+ by quercetin may have potentially reduced the cytotoxic effects of AgNPs produced in this study.
This is the first report on characterisation of M. oleifera leaf derived AgNPs using sunlight in terms of their MIC bioactivities. Antimicrobial screening of AgNPs revealed strong inhibition of gram-negative and gram-positive bacteria as well as various fungal species, irrespective of whether they were derived from F or FD leaf samples. More specifically, MIC values were recorded in the range 12.5–25 μg ml−1 for bacterial strains and 6.25 μg ml−1 for fungal strains.
The effective inhibition of both gram-negative and gram-positive bacteria by AgNPs derived from M. oleifera leaf extracts is of great significance as it demonstrates their broad-spectrum antibacterial activity. It also indicates that the mode of action is not affected by the difference in membrane stabilities of the bacteria since gram-positive bacteria contain a thick peptidoglycan layer whilst gram-negative bacteria possess a rigid outer membrane structure composed of lipids and lipoproteins. Contrasting results were observed in a similar study where AgNPs (5–35 μg ml−1) synthesised from reducing agents such as D-glucose and hydrazine, displayed effective activity towards gram-negative bacteria but minimal activity against gram-positive bacteria [61]. These findings are in accordance with recent studies that report a greater sensitivity of gram-negative bacteria to AgNPs, when compared to gram-positive bacteria [39, 62]. Additionally, in the previously mentioned study on AgNPs derived from M. oleifera leaf powder, no activity against K. pneumoniae was noted. This might be due to the fact that AgNPs in the present study are ±6 times smaller than those used in the previous study [43]. It is worth mentioning that E. faecalis displayed resistance to the commercial antibiotic neomycin but was susceptible to both AgNPs prepared in this study. This is significant since this bacterium has previously been reported for its intrinsic resistance and further acquisition of resistance genes leading to the emergence of E. faecalis as a nosocomial pathogen that is a refractory to most therapeutic agents [63]. In addition, the conjugative transfer of high levels of resistance genes from E. faecalis to S. aureus is well established and may probably explain the incidence of S. aureus resistance to neomycin observed in this study [64].
5. Conclusion
This study showed an innovative way to produce AgNPs with desirable physical attributes from FD and F leaf extract samples of M. oleifera, by the use of sunlight irradiation as an alternative energy source. The AgNPs produced were comparable between tissue samples in terms of their yield, morphology and bioactivities and showed that freeze-drying of the leaf material may be useful in their preservation for future nanoparticle synthesis. Significantly, biosynthesised AgNPs showed a broad-spectrum antimicrobial susceptibility range and therefore represent promising antimicrobial agents with potential biomedical applications.
Acknowledgments
This study was made possible through financial support from the National Research Foundation. The research facilities were provided by the University of KwaZulu-Natal.