Abstract
Three different kinds of plastic bags HL, VHL, and VN1 with different chemical nature were degraded by a novel thermophilic bacterial strain isolated from composting agricultural residual in Vietnam in shaking liquid medium at 55 °C after 30 d. The new strain was classified in the Bacillus genus by morphological property and sequence of partial 16Sr RNA coding gene and named as Bacillus sp. BCBT21. This strain could produce extracellular hydrolase enzymes including lipase, CMCase, xylanase, chitinase, and protease with different level of activity in the same media. After a 30-d treatment at 55 °C with Bacillus sp. BCBT21, all characteristics including properties and morphology of treated plastic bags had been significantly changed. The weight loss, structure and surface morphology of these bags as well as the change in the average molecular weight of VHL bag were detected. Especially, the average molecular weight of VHL bag was significantly reduced from 205 000 to 116 760. New metabolites from the treated bags indicated biodegradation occurring with the different pathways. This finding suggests that there is high potential to develop an effective integrated method for plastic bags degradation by a combination of extracellular enzymes from bacteria and fungi existing in the composting process.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Plastics have become omnipresent in our daily life because of low cost, excellent oxygen/moisture barrier properties, bio-inertness, and light weight, making them outstanding packaging materials. The plastics industry has grown continuously over the last 50 years and the global plastics production has reached 322 million tons in 2015 [1]. The global production of polyethylene terephthalate (PET) was 50.01 million tons just in 2016 and is expected to surpass 87 million tons by 2022 [2]. After a short first–use cycle, 95% of plastic package material value, or USD 80–120 billion, is lost annually [3]. The accumulation of plastic waste can cause severe damage to the environment and human life. Biodegradable plastics have been developed as an alternative to petrochemical-based plastics [4, 5]. However, both the production scale and usage of biodegradable plastics are still very limited due to their low durability and lack of supporting systems [6]. In Vietnam, the amount of solid waste generated has reached 44 million annually, and the figure is estimated to increase by 200% after each year. Several technologies have been developed to counter the plastic problem in Vietnam dealing with enhancing oxidation capability of plastics itself in parallel with creating biological products from natural resources for effectively plastic degradation.
Numerous thermophilic microbes have shown high potential for plastic degradation due to their ability to grow in different conditions and produce numerous oxidation and hydrolase enzymes [7–18]. The efficiency of biodegradation by this group of microbes depended on the physical, chemical and biological factors of treatment, including the enzymes involved in the degradation of plastic polymer, the metabolic pathways of uptaking the plastic fragments, and advantageous or inhibitory chemical factor to the biodegradation process [19]. Our study aims to develop an eco-friendly method by exploring the potential of thermophilic bacteria isolated from the agricultural composting residual and their enzymes for biodegradable and oxo-biodegradable plastic degradation.
2. Material and method
2.1. Material
The bacteria strain BCBT21 was isolated from the thermophilic phase of agricultural waste composting in Vietnam. Three groups of plastics used for biodegradability evaluation were: (i) plastic bags containing nano-additives from Netherlands (HL); (ii) oxo-biodegradable plastic bags produced by Institute of Chemistry (IoC) of Vietnam Academy of Science and Technology (VAST) which contained linear low-density polyethylene (LLDPE) with the concentration of more than 70% and high density polyethylene (HDPE) with the concentration of less than 30%; (iii) plastic bags with additives sold at supermarkets in Vietnam (VN1) which was claimed to be environmentally friendly.
2.2. Treatment process
Bacterial strain BCBT21 was incubated in 250 ml flask containing 150 ml medium composition follows (g l−1): 5 g peptone, 1 g glucose, 5 g NaCl, 3 g meat extract at pH 6.8 on a rotary shaker at 55 °C and 200 rpm for 3 d. Biodegradation treatment was performed with 1 gram of plastics bags (1.5 × 10 cm2) added to Erlenmeyer flasks individually. Then cultures were incubated on a rotary shaker at 55 °C and 200 rpm for 30 d.
2.3. Identification of bacterial isolate
The new bacterial strain was characterized by 16S rRNA fragment using the universal primers 27F (5'—AGA GTT TGA TCC TGG CTC AG—3') and 1492R (5'—TAC GGG TAC CTT GTT ACG ACT T—3') [20]. The sequence was corrected using FinchTV and compared with other sequences from GenBank (the National Center for Biotechnology Information (NCBI) database) using basic local alignment search tool (BLAST) analysis. The phylogenetic tree was made using MEGA 7 [21].
2.4. Screening for the extracellular enzyme activity
The activity of chitinase, carboxymethyl cellulase (CMCase), protease, lipase, xylanase in agar medium containing chitin, carboxymethyl cellulose (CMC), casein, tributyrin, xylan, respectively, as the substrates. Samples were collected at the starting point, and after 7, 14, 21, and 30 d. The activities were measured by the diameter of the clear zone on the substrate agar petri disks using 1% Lugol dye after incubating enzymes at 55 °C for 24 h.
2.5. Evaluation of surface structure of plastic bag
The morphology of treated and untreated plastic bags was characterized by scanning electron microscopy (SEM) using a JSM-6510 LV instrument (Jeol, Japan) at Institute for Tropical Technology, VAST and field emission scanning electron microscopy (FESEM) using a Hitachi S-4800 machine (Japan) at Vietnam National Institute of Hygiene and Epidemiology. For the structural morphology observation of the plastic bags, their surface was coated with a thin layer of platinum to conduct electricity and taken in a nitrogen atmosphere.
2.6. Determination the weight loss of the plastic bags
A simple and quick method to measure the biodegradation of plastic bags was determined by the weight loss of plastic bags after incubation. The weight loss of plastic bags was calculated and based on their weight change according to ASTM D6003-96 [22] by the formula:
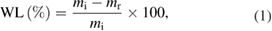
with WL is the weight loss of samples (%), mi is the initial sample weight (g), and mr is the retention weight of samples after testing (g).
2.7. Evaluation of chemical structure of the plastic bags
Fourier transform infrared spectroscopy (FTIR) analysis was performed by using Thermo Nicolet NEXUS 670 FTIR Spectrometer at room temperature to investigate the changes in chemical structure of the plastic bags with Bacillus sp. BCBT21. Each sample was recorded with 16 scans at a resolution of 4 cm−1 in the scanning range from 4000 to 400 cm−1 at Institute for Tropical Technology, VAST. To evaluate quantitatively the carbonyl group content changed during the testing of plastic bags, carbonyl index (CI) was measured by the following equation:

where  is the absorbance of the carbonyl stretching vibration,
is the absorbance of the carbonyl stretching vibration,  is the absorbance of symmetrical bending vibration of the methyl group, in which the absorbance of this group is nearly not changed during the testing process.
is the absorbance of symmetrical bending vibration of the methyl group, in which the absorbance of this group is nearly not changed during the testing process.
2.8. Change in tensile of plastic bags
Tensile strength and elongation at break of the untreated and treated plastic bags were conducted on Zwick instrument (Germany) at Institute for Tropical Technology, VAST according to ASTM D638 standard [23]. Each sample was measured five times to obtain the average value.
2.9. Color change of plastic bags
The color parameters of the untreated and treated plastic bags were determined by a ColorTecPCM (PSM™, USA) at Institute for Tropical Technology, VAST according to ASTM D2244-89 standard [24]. The total color difference (ΔE) of the samples was calculated using the following equations
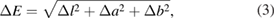
where
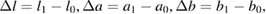
l is a measurement of brightness (Δl > 0 for light, Δl < 0 for dark), a is a measurement of redness or greenness (Δa > 0 for red, Δa < 0 for green), b is a measurement of yellowness or blueness (Δb > 0 for yellow, Δb < 0 for blue). l1, a1 and b1 are the color parameters of the tested sample; l0, a0 and b0 are the color parameters of the original sample. For each sample the color parameters were measured at ten different positions to obtain the average value.
2.10. Change in average molecular weight
The average molecular weight of VHL plastic bag containing PE main component was determined based on the data from viscosity measurement of the polymer solution. The sample was dissolved in xylene solvent at 105 °C, then non-polymer part (not dissolved in solvent) was filtered to obtain a polymer solution. The polymer solution viscosity was measured by using Ubbelohde Capillary Viscometer. The average molecular weight of the polymer was calculated by using solution intrinsic viscosity—polymer average molecular weight relationship according to the Mark–Houwink–Sakurada equation shown as follows [25]

where [η] = intrinsic viscosity and  is average molecular weight.
is average molecular weight.
2.11. Determination of the metabolites from the treatment of plastic bags
The metabolites received from the treatment of the plastics bags were determined according to the guideline for determination of volatiles and semi-volatiles by gas chromatography–mass spectrometry (GC-MS).
3. Results and discussion
3.1. Bacterial classification
Bacterial strain BCBT21 isolated from the thermophilic phase of agricultural waste was capable of growing well at 37, 55, and 65 °C. Colonial and cell morphologies of strain BCBT21 was shown in figure 1. Based on the partial 16S rRNA gene sequence, the thermophilic strain BCBT21 was closely related to Bacillus licheniformis strain DS42 and CAU7827. Based on colonial, cell morphologies, and the sequence of the 16S rRNA coding gene, the strain BCBT21 was classified to Bacillus genus and designed as Bacillus sp. BCBT21. The partial 16S rRNA sequence of Bacillus sp. BCBT21 was deposit in GenBank under ID KX815277.1.
Figure 1. Colonial (a) and cell (b) morphology of the strain BCBT21.
Download figure:
Standard image High-resolution image3.2. Weight loss of plastic bags by Bacillus sp. BCBT21
After 30 d of treatment at 55 °C by Bacillus sp. BCBT21 in liquid shaking culture, the weight loss of the plastics was identified with different rates: 60.75 ± 7.26% of 1 g HL, 11.00 ± 1.00% of 1 g VHL and 4.44 ± 0.45 of 1 g VN1 (table 1). The treated HL plastic bag had the weight loss five and ten times compared with VHL and VN1 plastic bags in the same condition. The degradability of three plastic bags by the Bacillus sp. BCBT21 was in following order: HL > VHL > VN1. The VHL plastic bag had weight loss much higher than the VN1 plastic bag because of the difference in the chemical nature of these two plastic bags as mentioned at materials section 2.1. In contrast, VN1 sold at the supermarket was fabricated from HDPE with biodegradable additive amendment (claimed by producer: it is an imported technology with certification 'Environmental friendly plastic bags'). The LLDPE was known to be easier biodegraded than HDPE.
Table 1. Weight loss of HL, VHL and VN1 plastic bags after 30 d treatment in shaking cultivation by Bacillus sp. BCBT21.
| Plastic bag | HL | VHL | VN1 |
|---|---|---|---|
| Weight loss (%) | 60.75 ± 7.26 | 11.00 ± 1.00 | 4.44 ± 0.45 |
Weight loss of different plastics treated by bacteria isolated from various sources did not show the same efficiency. Three Pseudomonas strains isolated from sewage sludge sump, household garbage dump, and textile effluent drainage were capable of degrading up to 46.2% natural polyethene and 29.1% synthetic polyethene at 40 °C [26]. After 60-d cultivation at 33.3 °C, two strains Bacillus amyloliquefaciens BSM-1 and Bacillus amyloliquefaciens BSM-2 isolated from municipal solid waste (MSW) soil degraded 30 mg LDPE in 60 d with 11% and 16% weight loss, respectively [27]. The plastic degradation efficiency of Pseudomonas putida in the one-month period was 30% weight loss which is much higher in comparison with others. Lowest degradation rate was observed in case of B. subtilis (22% weight loss per one month) [28]. Weight loss/month of polythene bag treated by two strains B. amylolyticus and B. subtilis using liquid shaking culture method was 30% and 20%, respectively [18]. B. cereus isolated from MSW compost degraded LDPE, and BPE10 with 17.036% weigh loss [17]. Another Bacillus cereus isolated from dumpsite soil degraded 7.2−2.4% polyethylene [29]. Brevibaccillus borstelensis strain isolated from soil could reduce gravimetric and molecular weights of low-density by 11 and 30%, respectively, after 30 d at 50 °C [30]. Four novel bacteria isolated from cow dung identified as B. vallismortis bt-dsce01, P. protegens bt-dsce02, Stenotrophomonas sp. bt-dsce03, and Paenibacillus sp.bt-dsce04 were capable of degrading LDPE strips and pellets, HDPE strips and pellets with the rate 75 ± 2, 55 ± 2, 60 ± 3, and 43 ± 3%, respectively, for a period of 120 d at 55 °C. The weight loss of plastics by bacteria and actinomycetes that isolated, cultivated from different environments and conditions listed above in comparison to Bacillus sp. BCBT21 strain in this study was much lower. The thermophilic bacterial Bacillus sp. BCBT21 has a high efficiency of HL bag degradation at 55 °C in liquid cultivation with 1 gram plastic bag at the initial point. The lowest degrading efficiency was environmental friendly plastic bag VN1 (table 1).
3.3. Extracellular enzymes activity during the treatment
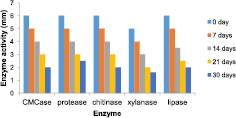
Hydrolase enzymes from Bacillus sp. BCBT21 were detected at 55 °C during the treatment. These enzymes were extracellular including CMCase, protease, chitinase, xylanase, and lipase with low activity and the activities were decreased over the treatment time (figure 2). High temperature also takes part in the plastic degrading process as a thermal factor.
Figure 2. Extracellular enzymes during 30-d treatment by Bacillus sp. BCBT21.
Download figure:
Standard image High-resolution imageBacterial and fungal strains isolated from different source showing high plastic degradation capacity by oxidoreductase and hydrolase enzyme combination with pretreatment was reported. Streptococcus, Bacillus, Pseudomonas, Staphylococcus, Aspergillus, Penicillium, Phanerochaete, Pestalotiopsis degrade plastics by inducing laccase, cutinase, hydrolase, esterase, urease, and the factors influenced to the enzyme production [12]. The depolymerisation process carried out by depolymerising amylase, lignin/manganese peroxidase, laccase and other hydrolase enzyme producing by potential bacteria and fungi isolated from plastic waste dump yard sites. These microbes are potential candidates for polyethylene bioremediation [15]. Lipases could still display a certain activity against PET even with the limited the access to the substrates because of the PET lid structure [31–33]. Ureases, esterases and proteases could be able to depolymerize polyurethane by hydrolyzing the urethane and ester bonds [34–39]. In this study hydrolase enzyme such as CMCase, protease, chitinase, xylanase and lipase induced by Bacillus sp. BCBT21 during the treatment suggests that they play a certain role in plastic bag degradation.
3.4. Morphology of plastic bags
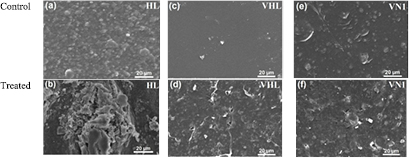
As mentioned in ASTM sub-committee D20-96 (ASTM standards pertaining to the biodegradability and compost ability of plastics), surface damage may be evaluated using effective tools such as SEM and FESEM method [40]. The SEM images of the untreated and treated plastic bags were shown in figure 3. At the magnification of 1000 times, it can be seen that the structure of untreated plastic bag is heterogeneous in two phases: a nano-additive dispersed phase with the size of 100 nm–1 µm and a plastic matrix phase. The surface deterioration of HL plastic bags is large with cavities, cracks, disintegration and holes while the surface of VHL and VN1 plastic bags have some small cracks, holes, and pits after 30 d of incubation with Bacillus sp. BCBT21 when compared with the control samples. These changes indicated that biodegradation processes of all plastic bags had occurred. The Bacillus sp. BCBT21 can break down the polymer chains in HL plastic bag more efficiently than that in VHL and least efficiently in VN1 plastic bags. In particular, all tested plastic bags contain the nano-additives which may enhance degradation process of these plastic bags.
Figure 3. SEM images of untreated and treated HL ((a) and (b)), VHL ((c) and (d)) and VN1 ((e) and (f)) plastic bags, respectively, by Bacillus sp. BCBT21.
Download figure:
Standard image High-resolution imageTo observe the destroyed structure of treated plastic bags more clearly, the FESEM images of treated plastic bags were taken at the magnifications of 10 000 and 50 000 times (figure 4). From figure 4, it was seen that the plastic fragments in size of 2 µm for treated HL plastic bag. The appearance of large cracks with the length of 5 µm and diameter of 50 nm could be observed for treated VHL sample, while the 100−500 nm diameter circle holes are found in the treated VN1 plastic bag. This confirmed that the Bacillus sp. BCBT21 have been able to break down the tested plastic bags into lower molecular weight substances [41, 42]. Das and Kumar suggested that plastics could also be degraded by the creation of bio-film [43]. The degradation of plastic only started after the Bacillus began colonizing the plastic sheets by utilizing polymers as the sole source of carbon. The biodegradation of plastic or polyolefin basing on the help of enzymes also was reported in some research, for example, in the case of polyethylene [44] and plastic film [45].
Figure 4. SEM images of treated HL ((a) and (b)), VHL ((c) and (d)) and VN1 ((e) and (f)) plastic bags by Bacillus sp. BCBT21 at magnifications of 10 000 ((a), (c) and (e)) and 50 000 ((b), (d) and (f)).
Download figure:
Standard image High-resolution image3.5. FTIR spectra of plastic bags
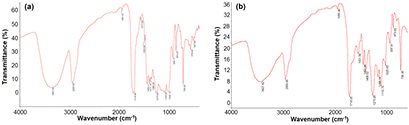
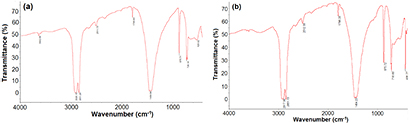
FTIR was used for evaluation of changes in material chemical structure through the degradation process of the polymer. From FTIR spectrum of HL plastic bag (figure 5), a broad peak was found at 3367.50 cm−1 corresponding to the stretching vibration of OH group with hydrogen bonding or CONH or NH or C≡C–H groups in the polymer. The absorbance range of 3500–3200 cm−1 corresponded to the presence of alcohols and phenols [46]. Several peaks found at 2955.56 cm−1, 1389.45−1455.11 cm−1 and 730.29 cm−1 were characterized for stretching, bending and out-plane vibrations of CH group in the polymer chain. The absorbance range of 1500–1400 cm−1 corresponded to –CH2 stretching and presence of aromatics in polymer chain [47].
Figure 5. FTIR spectra of the (a) untreated and (b) treated HL plastic bags with Bacillus sp. BCBT21.
Download figure:
Standard image High-resolution imageThe stretching vibration of C=O group in acid or ketone moiety in the polymer appeared at 1716.96 cm−1. Also, the peak of C–O group in the polymer was exhibited in the range of 1026.12 cm−1 to 1273.04 cm−1 while the peak of NH group could be observed at 1577.96 cm−1. Several other peaks could be demonstrated for the nano-additives introduced into the plastic. From the above characteristics, HL plastic bag could be fabricated by several polymers such as polylactic acid, polyvinyl alcohol, polycaprolactone.
In comparison with the original HL plastic bag, the FTIR spectrum of HL treated bag expressed many different points. The intensity and wave numbers of most of the corresponding peaks were shifted remarkably. For instance, the peak of OH group was changed from 3427.42 cm−1 to 3367.50 cm−1. The peaks of CH, NH and C=O groups were also shifted from 2 to 19 cm−1. Interestingly, the peaks of nana-additives at 579.43 and 527.59 cm−1 could not be observed in the FTIR spectrum of HL treated bag, which could be the result of the dissolve of nana-additives into the environment during thermophilic bacterial cultivation process. Moreover, the clear decrease in the intensity of C−O and CH2 group could confirm that the Bacillus sp. BCBT21 had a favor to break down the C−O and aromatic groups as well as alcohols and phenols. Furthermore, the new peak found at 873.82 cm−1 was corresponded to –C=C– stretching and the presence of alkene group [48]. The result was the proof that the HL plastic bag was degraded by the Bacillus sp. BCBT21 after 30 d cultivation. The dissolve of nano-additives into cultivating liquid phase with the extracellular enzyme might play a role in the improvement of degradation process of HL plastic bag.
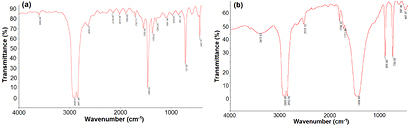
Based on the FTIR spectra of untreated and treated VHL and VN1 plastic bags (figures 6 and 7), it was clear that the shapes and the peaks of characterized groups in these bags are quite similar. There were only some main peaks characterized by stretching, bending and out-plane vibrations of CH groups at 2850−2928 cm−1, 1464 cm−1 and 728 cm−1. Other peaks corresponded to the vibration of some groups in the nano-additives in the plastic bags. This observation suggested that the nature of these plastic bags is hydrocarbon chain polymers of polyethylene.
Figure 6. FTIR spectra of the (a) untreated and (b) treated VHL plastic bags with Bacillus sp. BCBT21.
Download figure:
Standard image High-resolution imageFigure 7. FTIR spectra of the (a) untreated and (b) treated VN1 plastic bags with Bacillus sp. BCBT21.
Download figure:
Standard image High-resolution imageThe position and intensity of main characterized peaks of polymer and other peaks of nano-additives in the FTIR spectra of the treated VHL and VN1 plastic bags were fixed. However, a small shift from 5 to 10 cm−1 for characterized peaks of out-plane vibration of CH group in polymers and for stretching vibration of OH or NH group in nano-additives. The difference between the FTIR spectra of VHL and VN1 plastic bags was the existence of a new peak found at 1711.80 cm−1 corresponding to the stretching vibration of C=O group in acid, ester or ketone in the VHL bag. The result indicated that the structure of polymers was almost unchanged and nano-additives in plastic bags could be dissolved. Thus, the effect of Bacillus sp. BCBT21 on VHL plastic bag was more than that on the VN1 plastic bag. The polymer chain in VHL plastic bag could be break down by the Bacillus sp. BCBT21 and form lower molecular weight substances like ketone, acid or ester. These additives accelerated photo-oxidation degradation of polymer macromolecules to form a polymer with lower molecular weight and oligomer containing oxygen groups such as carbonyl, carboxylic, hydroperoxide. The carbonyl index (CI) of three plastic bags was calculated by equation (2) basing on the FTIR spectra. It was reduced gradually to HL plastic bag and increased insignificantly for VHL and VN1 plastic bags after 30th day treating by Bacillus sp. BCBT21 as demonstrated in table 2. The decrease in the CI value of HL plastic bag confirmed that oxidized polymers had been utilized by the microorganisms [48].
Table 2. Characteristics, properties of untreated and treated HL, VHL and VN1 plastic by Bacillus sp. BCBT21 cultivation.
| Characteristics and properties | Control | Treated | |||||
|---|---|---|---|---|---|---|---|
| HL | VHL | VN1 | HL | VHL | VN1 | ||
| Carbonyl index (CI) | 5.51 | 0.01 | 0.01 | 2.91 | 0.02 | 0.02 | |
| Tensile strength (MPa) | 8.39 | 24.25 | 15.41 | UD | UD | UD | |
| Elongation at break (%) | 14.82 | 180.21 | 585.22 | UD | UD | UD | |
| Total colour difference (ΔE) | — | — | — | UD | UD | 4.01 | |
Note: UD is unidentified due to break down of plastic bags formed small pieces.
The FTIR result indicated chemical structural alterations in treated HL and VHL plastic bags by Bacillus sp. BCBT21 with the appearance of new plastic functional groups (table 3). This finding showed that the chemical structure of these plastic bags had changed by different pathway depending on the chemical nature.
Table 3. FTIR indication of chemical structural alterations in treated plastic bags by Bacillus sp. BCBT21.
| Plastic bags | Control | Treated | New group |
|---|---|---|---|
| VN1 | OH, NH, CH | OH, NH, CH | — |
| VHL | OH, NH, CH | OH, NH, CH, C=O | C=O |
| HL | OH, CONH, N–H, CH, CH2, C≡CH, C=O, C–O | OH, CONH, N–H, CH, CH2, C≡CH, C=O, C–O, –C=C– | –C=C– |
3.6. The change in tensile properties of plastic bags
The tensile properties of the untreated and treated plastic bags were determined according to ASTM D638 standard and listed in table 2. The tensile strength and elongation at break of original HL plastic bag were quite low, 8.39 MPa and 14.82%, respectively. The original VHL plastic bag has tensile strength higher and elongation at break lower than the original VN1 plastic bag. The HL and VHL plastic bags after 30 d treating in bacterial cultivation were broken up into a lot of small pieces and unable to determine the mechanical properties of these plastic bags. The VN1 treated plastic bag maintained a suitable size for testing but was broken up immediately when applied stress force. The results of tensile properties indicate that the plastic bags were degraded by the Bacillus sp. BCBT21.
3.7. The change in the colour of plastic bags
Colour characteristic is also used to quantify the degradation of the plastics in different conditions. For instance, ΔE > 0 corresponds to change in the colour of the plastic could indicate that the plastic was degraded in testing condition. Normally, some colour additives and oxo-degradable additives were added to plastic bags. When plastics were degraded, the colour additives and oxo-degradable additives would be released into the solution leading to a colour change of the surface of plastics [49]. In case of the HL and VHL incubated bags which were broken into small pieces, it was not able to calculate their colour parameters. For the VN1 treated bag, the obtained results shown in table 2 confirmed that the colour of this plastic bag has changed due to the ΔE > 0, indicated that it was degraded by Bacillus sp. BCBT21.
3.8. The change in average molecular weight
In this study VHL bag was chosen for average molecular weight detection to confirm the degrading action of thermophilic bacterium Bacillus sp. BCBT21. The average molecular weight of VHL plastic bag with PE as the main component was determined based on the data from viscosity measurement of the polymer solution. The average molecular weight  of VHL plastic bag was significantly decreased after treatment with Bacillus sp. BCBT21 at 55 °C (table 4). In this case, the high temperature and the induced extracellular hydrolase enzymes play an important degrading role of oxo-biodegradable VHL. Moreover, for oxo-degradable additives introduced into the VHL plastic bags, both high temperature accelerated oxidation and the photo-oxidation degradation were also involved in the degradation process. A polymer with low molecular weight and oligomer containing oxygen groups such as carbonyl, carboxylic, hydroperoxide groups were formed from the polymer macromolecules after the oxidation reaction. Low molecular weight polymer chain, then, can be attacked and hydrolyzed by enzymes from Bacillus sp. BCBT21 to form even lower molecular weight substances like alkyls, alkanes, esters. After treating with Bacillus sp. BCBT21. The
of VHL plastic bag was significantly decreased after treatment with Bacillus sp. BCBT21 at 55 °C (table 4). In this case, the high temperature and the induced extracellular hydrolase enzymes play an important degrading role of oxo-biodegradable VHL. Moreover, for oxo-degradable additives introduced into the VHL plastic bags, both high temperature accelerated oxidation and the photo-oxidation degradation were also involved in the degradation process. A polymer with low molecular weight and oligomer containing oxygen groups such as carbonyl, carboxylic, hydroperoxide groups were formed from the polymer macromolecules after the oxidation reaction. Low molecular weight polymer chain, then, can be attacked and hydrolyzed by enzymes from Bacillus sp. BCBT21 to form even lower molecular weight substances like alkyls, alkanes, esters. After treating with Bacillus sp. BCBT21. The  of VHL bag was reduced 43.05% from 205 000 to 116 760. This result once again confirmed the previous changes in VHL plastic morphology, weight loss, chemical structure.
of VHL bag was reduced 43.05% from 205 000 to 116 760. This result once again confirmed the previous changes in VHL plastic morphology, weight loss, chemical structure.
Table 4. Average molecular weight ( ) of untreated and treated VHL plastic bags in the same conditions.
) of untreated and treated VHL plastic bags in the same conditions.
| Sample | Intrinsic viscosity [η]) | 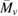 |
|---|---|---|
| VHL-control | 0.2201 | 205 000 |
| VHL-BCBT21 | 0.1384 | 116 760 |
3.9. Metabolites from the treatment of plastic bags by Bacillus sp. BCT21
GC-MS analysis showed that new metabolites were formed from HL, VHL and VN1 plastic bags after degraded by Bacillus sp. BCBT21 (appendix). The results of the metabolites found in treated and untreated samples with Bacillus sp. BCBT21 revealed that there were more metabolites found in treated HL and VHL samples than the treated VN1 sample. This demonstrated that the ability of decomposing of VN1 sample was worse than that of HL and VHL samples. More than one-third of total compounds found in treated HL and VHL samples were the same. Metabolites found in the treated VN1 sample were different in chemical structures with just one same metabolite with treated HL and VHL samples. This evidence showed that the mechanisms of structural transformation of HL and VHL plastic bags were similar. The chemical structures of the VN1 plastic bag were distinctly different from that of HL and VHL. There were benzene rings and furan heterocycles in the chemical structures of the metabolites in the HL sample while there were only furan heterocycles detected in untreated VHL and VN1 samples. However, after treatment with thermophilic bacteria, both HL and VHL samples showed the presence of phenols and nitrogen heterocyclic six-membered ring compounds such as pyrudin, pyrazine and quinazoline. In VN1 sample, nitrogen heterocyclic five-membered ring compounds such as pyrols and indols were found after treatment. This finding, once again, confirmed that the mechanisms of degradation by Bacillus sp. BCBT21 of HL and VHL samples were similar, while the mechanism of VN1 degradation might be completely different.
4. Conclusion
A thermophilic bacteria Bacillus sp. BCBT21 isolated from composting agricultural residual was capable of degrading biodegradable and oxo-biodegradable plastics from various resources. Hydrolases secreted by Bacillus sp. BCBT21 including chitinase, CMCase, protease, xylanase, and lipase acting in the high temperature might play an important role in plastic degradation. The biodegradable plastic bag selling at the supermarket in Vietnam appeared to be more difficult to degrade when compared with the one selling in Netherlands and the plastic bag developed by VAST under the same treatment condition. This study revealed the potential of the microorganism consortium and their enzymes in the mini-ecosystem such as agriculture waste composting residual in plastic degradation. The result also exposed the differences in three kinds of biodegradable plastic bags to develop a more environmentally friendly product in the future.
Acknowledgment
The authors acknowledge the financial support provided by Vietnam Academy of Science and Technology (2015-2017) for the project 'Biodegradability evaluation of biodegradable polymers which have been using in Vietnam under different environmental treatments'.
Appendix. Metabolites from the treatment of plastic bags by Bacillus sp. BCT21 after 30 d
| Plastic bag | Control | After treatment | New metabolites |
|---|---|---|---|
| HL | Hexadecane, 1,1-bis(dodecyloxy)-; 1-Hexadecanol,2-methyl-; 2-Cyclohexen-1-one, 2,4,4-trimethyl-3-(3-oxo-1-butenyl)-; 1,2-Benzenedicarboxylic acid, butyl octyl ester; Tetracyclo[6.2.2.2(4,9).0(4,10)]tetradecan-2-one, 10,12-dihydroxy-1,3,7,8-tetramethyl-; 4-Methylandrost-4-en-17-ol-3-one acetate; 3,5a,9-Trimethyl-3a,5,5a,9b-tetrahydro-3H,4H-naphtho[1,2-b]furan-2,8-dione; Eudesma-5,11(13)-dien-8,12-olide; 2-Propanol, 2-methyl-; 1H-Cyclopenta[c]furan-3(3aH)-one, 6,6a-dihydro-1-(1,3-dioxolan-2-yl)-, (3aR,1-trans,6a-cis)-; 2-Trifluoroacetoxydodecane; Propane, 2-ethoxy-2-methyl-; Amylene hydrate; Furan, tetrahydro-2,2,5,5-tetramethyl-; 3-Trifluoroacetoxypentadecane; Orthoformic acid, tri-sec-butyl ester; | Hexadecane, 1,1-bis(dodecyloxy)-; 1-Hexadecanol, 2-methyl-; 6-Methyl-5-oxo-11-propenyl-12,13-dioxa-tricyclo[7.3.1.0(1,6)]tridecane-8-carboxylic acid; Ethanone, 1-(6-methyl-3-pyridinyl)-; Quinazoline, 4-methyl-; 1-Propanone, 1-(4-aminophenyl)- | 6-Methyl-5-oxo-11-propenyl-12,13-dioxa-tricyclo[7.3.1.0(1,6)]tridecane-8-carboxylic acid; Ethanone, 1-(6-methyl-3-pyridinyl)-; Quinazoline, 4-methyl-; 1-Propanone, 1-(4-aminophenyl)- |
| Phenol, 2,4-bis(1,1-dimethylethyl)-; 2-Ethyl-3-methyl-6,7-dihydro-5H-cyclopenta[b]pyridine-4-amine; 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo[1,2-a:1',2'-d]pyrazine; 1-Gala-1-ido-octonic lactone; 1-Monolinoleoylglycerol trimethylsilyl ether; 2-Trifluoroacetoxydodecane; Propane, 2-ethoxy-2-methyl-; Amylene hydrate; Furan, tetrahydro-2,2,5,5-tetramethyl-3-; Trifluoroacetoxypentadecane | |||
| Phenol, 2,4-bis(1,1-dimethylethyl)-; 2-Ethyl-3-methyl-6,7-dihydro-5H-cyclopenta[b]pyridine-4-amine; 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo[1,2-a:1',2'-d]pyrazine; 1-Gala-1-ido-octonic lactone; 1-Monolinoleoylglycerol trimethylsilyl ether; 2-Propanol, 2-methyl-; 1H-Cyclopenta[c]furan-3(3aH)-one, 6,6a-dihydro-1-(1,3-dioxolan-2-yl)-, (3aR,1-trans,6a-cis)-; Propane, 2-ethoxy-2-methyl-; Amylene hydrate; Propane, 2-(ethenyloxy)-; Furan, tetrahydro-2,2,5,5-tetramethyl-; Pyrazine, 2,5-dimethyl-; 2-Heptanone, 4-methyl-; Orthoformic acid, tri-sec-butyl ester | |||
| VHL | 2-Cyclohexen-1-one, 2,4,4-trimethyl-3-(3-oxo-1-butenyl)-; 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione; Heptacosane; 2-Propanol, 2-methyl-1H-Cyclopenta[c]furan-3(3aH)-one, 6,6a-dihydro-1-(1,3-dioxolan-2-yl)-, (3aR,1-trans,6a-cis)- | Ethanone, 1-(2-aminophenyl)-; Quinazoline, 4-methyl-; 1-Propanone, 1-(4-aminophenyl)-; Phenol, 2,4-bis(1,1-dimethylethyl)-; Pyrazine, 2-butyl-3,5-dimethyl-; N,N'Bis(Carbobenzyloxy)-lysine methyl(ester); 1H-Inden-1-one, 5-amino-2,3-dihydro-; 1,2 Benzenedicarboxylic acid, butyl octyl ester; Benzoisofur 2-Propanol, 2-methyl- | Ethanone, 1-(2-aminophenyl)-; Quinazoline, 4-methyl-; 1-Propanone, 1-(4-aminophenyl)-; Phenol, 2,4-bis(1,1-dimethylethyl)-; Pyrazine, 2-butyl-3,5-dimethyl-; N, N'-Bis (Carbobenzyloxy)-lysine methyl(ester); 1H-Inden-1-one, 5-amino-2,3-dihydro-; 1,2-Benzenedicarboxylic acid, butyl octyl ester; Benzoisofuran-1-one, 1,3-dihydro-6,7-dimethoxy-3-(4,5,6,7-tetrahydrobenzothiazol-2-yl)amino-; Disulfide, dimethyl; Pyrazine, 2,5-dimethyl-; Orthoformic acid, triisobutyl ester |
| Propane, 2-ethoxy-2-methyl- | |||
| Amylene hydrate Furan, tetrahydro-2,2,5,5-tetramethyl- | |||
| Arginine | |||
| 1H-Cyclopenta[c]furan-3(3aH)-one, 6,6a-dihydro-1-(1,3-dioxolan-2-yl)-, (3aR,1-trans,6a-cis)- | |||
| Propane, 2-ethoxy-2-methyl- | |||
| Amylene hydrate | |||
| Disulfide, dimethyl | |||
| Pyrazine, 2,5-dimethyl- | |||
| Orthoformic acid, triisobutyl esteran-1-one, 1,3-dihydro-6,7-dimethoxy-3-(4,5,6,7-tetrahydrobenzothiazol-2-yl) amino-; | |||
| VN1 | 4-(1,5-Dihydroxy-2,6,6-trimethylcyclohex-2-enyl)but-3-en-2-one; 2-Cyclohexen-1-one, 2,4,4-trimethyl-3-(3-oxo-1-butenyl)-; 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione; Propanoic acid, 2-methyl-, (dodecahydro-6a-hydroxy-9a; 1,2,3,4-Tetrahydro-7-methoxy-3,8-dimethyl-9H-carbazol-1-one; 2-Propanol, 2-methyl-; 1H-Cyclopenta[c]furan-3(3aH)-one, 6,6a-dihydro-1-(1,3-dioxolan-2-yl)-, (3aR,1-trans,6a-cis)-; Propane, 2-ethoxy-2-methyl-; Amylene hydrate; Furan, tetrahydro-2,2,5,5-tetramethyl-; 2,5-Dimethylhexane-2,5-dihydroperoxide; Orthoformic acid, tri-sec-butyl ester | henol; 2-Piperidinone; Indole; Benzeneethanol, α-ethyl- | Phenol; 2-Piperidinone; Indole; Benzeneethanol, α-ethyl- |
| Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)-; Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)-; 2-Propanol, 2-methyl-; 1H-Cyclopenta[c]furan-3(3aH)-one, 6,6a-dihydro-1-(1,3-dioxolan-2-yl)-, (3aR,1-trans,6a-cis)-; Propane, 2-ethoxy-2-methyl-; 2-Butanone; 1-Propanol, 2-methyl-; Amylene hydrate; 2-Pentanone; Disulfide, dimethyl; | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)-; Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)-; 2-Butanone; 1-Propanol, 2-methyl-; 2-Pentanone; Disulfide, dimethyl | ||