Abstract
The different water soluble ink-jet inks based on conjugated poly (3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) were formulated with various organic co-solvents such as diethylene glycol (DEG), triethylene glycol (TRIEG), tetraethylene glycol (Tetra EG), and polyethylene glycol (PEG) to print on the flexible substrate. The effect of adding various organic co-solvents, which show a same trend of molecular weight, dipole moment and boiling point on the formulated inks properties and ink-jet printed films electrical conductivity, morphology and transparency were investigated. The conductivities of the ink-jet printed films were evaluated by four-point probe conductivity measurement. The morphologies of the printed films have been studied using atomic force microscopy and scanning electron microscopy. The size of PEDOT:PSS particles and the printed film morphology altered by addition of different types of co-solvent in the ink-jet ink formulation. The electrical conductivity of the ink-jet printed film increased by raising the molecular weight, boiling point and dipole moment of used co-solvent in ink-jet ink formulation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Polymeric materials, especially conducting polymers [1–5] are a promising candidate for application in various flexible optoelectronic devices such as light-emitting diodes, field effect transistors, and organic solar cells that are based on charge transport properties of the comprised materials.
The amorphous materials such as polymers due to their electronic, optical and mechanical properties and their ability for a low cost of device fabrication for instance solution process [6, 7] has been used instead of crystalline, brittle and expensive indium tin oxide (ITO) [8, 9], carbon nanotubes [10–12], graphene [13–16], metal nanowires [17–19], metal grids [20] and thin metals [21]. The solution process allows for the elimination of high vacuum and temperature processes such as vacuum evaporation, sputtering [2, 5] and the use of flexible substrates to produce flexible devices [22, 23]. Among a number of conducting polymers, an aqua dispersion of poly (3,4-ethylenedioxythiophene) (PEDOT) is the material most widely used in optoelectronic devices. One of the more common uses of PEDOT:PSS is in an antistatic layer in photographic films (AGFA-Gevaert NV) that need highly conductivity, whereas low conductivities are appropriate for the wide-spread use as a transparent anode electrode in flexible electronic devices or a hole-transport layer in organic light emitting diodes, conductivities in the range of  .
.
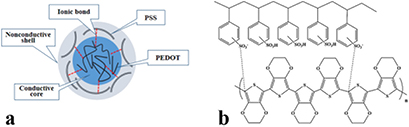
In the PEDOT:PSS, the PEDOT is a conductive p-type polymer that is not soluble in polar solvents such as water. Therefore, it doped with PSS, which is an ionic conductor to provide a stable, easy-to-process, deep blue water-based PEDOT:PSS dispersion [24]. The PSS in the complex functions as a counter ion for the cationic, conductive PEDOT. Although the addition of PSS brings an enhanced process-ability, this comes at the expense of a decreased electrical conductivity. A structural model of PEDOT:PSS is shown in figure 1. In this model, oligomer PEDOT segments are tightly, by ion interaction bond attached to PSS chains of much higher molecular weight and that coils up to form a tertiary structure [25–27]. The high conductivity of PEDOT:PSS dispersions can then be attributed to set arrangements of the PEDOT chains within a larger, tangled structure of loosely cross-linked, highly water-soluble PSS particles. The PEDOT:PSS can easily be fabricated by solution processes such as spin coating, dip coating, ink-jet printing and conventional printing to produce a thin, optically transparent, conductive films. In this study, the drop-on-demand (DOD) ink-jet printing that has grown to a major topic in scientific research due to their low price, low space demand, environmental safety, minimum chemical waste and ability to print any image or pattern from monitor on the desired location onto various substrates was chosen. Ink-jet printing is a non-contact method in which desired amount of ink droplets are directly deposited from very fine nozzles through a computer designed image onto a selected area of a substrate without any contamination. The two most popular types of DOD ink-jet printers are thermal and piezoelectric. Compared to conventional manufacturing method ink-jet printing can be carried out in low temperatures. Despite all the advantages of the ink-jet printing technology, nozzle clogging and achieving a uniform printed film has always been a major problem. The nozzle clogging occurs when the ink-jet printing inks contain insoluble micro or nanoparticles that can be agglomerated and precipitated during the printing process. The non-uniform printed film is due to either improper ink physical properties or drying time of printed film. Therefore, preparing the ink-jet ink is often a challenging issue for researchers.
Figure 1. The structural model of PEDOT:PSS.
Download figure:
Standard image High-resolution imageRecently, various studies have demonstrated to enhance the conductivity of spin coated or sprayed PEDOT:PSS onto the glass or PET substrates by the post-treatments with acids [28, 29], changing the PEDOT:PSS ratios [30], treatment of PEDOT:PSS with polar organic solvents such as alcohols [31] or the addition of an ionic liquid [32], an anionic surfactant [33] or solvents such as dimethyl sulfoxide [30], sorbitol [34], diethylene glycol [35] or ethylene glycol [36] into the PEDOT:PSS aqueous solution. Adding co-solvents include dielectric screening due to the solvent, conformational changes of the PEDOT chain or morphological changes [35, 37–39].
In this study, the various organic co-solvents, which have different structures, dipole moments and boiling points are added to the ink formulation to not only adjust the physical properties of ink-jet ink to improve the wettability of the polymer on the substrate but also to optimum the drying times of ink-jet printed film to achieve optimum conductivity and transparency.
2. Experimental
2.1. Material
The materials used in this experiment were: poly (3,4-ethylene dioxythiophene): polystyrene sulfonate (PEDOT:PSS) as 1.3 wt% dispersion in  (
( ) and ITO coated polyethylene terephthalate (PET) with surface resistivity
) and ITO coated polyethylene terephthalate (PET) with surface resistivity  were provided by Sigma-Aldrich; the various co-solvents such asdiethylene glycol (DEG), triethylene glycol (TRIEG), tetraethylene glycol (TETRAEG), polyethylene glycol 200 (PEG200) and all other related substances used in this work were chosen from laboratory grade as received from Merck Company (Germany). Table 1 shows the properties of the co-solvents used in the PEDOT:PSS ink formulations.
were provided by Sigma-Aldrich; the various co-solvents such asdiethylene glycol (DEG), triethylene glycol (TRIEG), tetraethylene glycol (TETRAEG), polyethylene glycol 200 (PEG200) and all other related substances used in this work were chosen from laboratory grade as received from Merck Company (Germany). Table 1 shows the properties of the co-solvents used in the PEDOT:PSS ink formulations.
Table 1. The properties of co-solvents used in the formulations.
| Ink | Name | Structure | Dipole moment (Debye) | Boiling point (°C) |
|---|---|---|---|---|
| 1 | Diethylene glycol (DEG) | 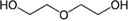 |
2.7 | 245 |
| 2 | Triethylene glycol(TRIEG) | 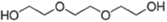 |
2.99 | 288 |
| 3 | Tetraethylene glycol (TRIEG) | 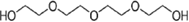 |
3.25 | 329 |
| 4 | Polyethylene glycol (PEG) | 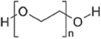 |
3.6 | >300 |
2.2. Equipment and instrumentation
The prepared ink formulations were filtered through a  and
and  Sartorius Minisart filter (Göttingen, Germany). The indium tin oxide coated PET were ink-jet printed using an Epson Stylus Photo P50 printer in 1 and 3 printing runs. Then, the printed flexible PET were dried and heat-treated in an Azar 1250 furnace at 130 °C [40]. The equilibrium contact angle
Sartorius Minisart filter (Göttingen, Germany). The indium tin oxide coated PET were ink-jet printed using an Epson Stylus Photo P50 printer in 1 and 3 printing runs. Then, the printed flexible PET were dried and heat-treated in an Azar 1250 furnace at 130 °C [40]. The equilibrium contact angle  of each ink/substrate mixture was measured using charge coupled device (CCD) camera and image analysis software (LB-ADSA mode). The pH, surface tension and viscosity of the ink were obtained using 827 pH Metrohm meters, Tensiometer K100MK2 and Anton Paar MCR 300 Rheometer, correspondingly. The surface morphology and cross sectional view of printed PEDOT:PSS ink on the flexible PET substrate was determined through the use of a LEO; model 1455VP scanning electron microscope (SEM) and DME Dual Scope C320 model atomic force microscopy (AFM). Additionally, morphology and size of the PEDOT:PSS inks were observed under a transmission electron microscopy (TEM) (JEM-100CXII). Particle size of PEDOT:PSS was determined by dynamic light scattering (DLS) equipment (Malvern Instrument, UK; model ZEN 3600), which measured the random changes in the intensity of light scattered from an ink. Optical transmittance of the ink-jet printed PEDOT:PSS films were measured, within the wavelength range of 300 – 800 nm, by means of a UV/visible spectrometer (Agilent 8453). Sheet resistance of the ink-jet printed PEDOT:PSS films was measured by a WS-1 type four-probe apparatus.
of each ink/substrate mixture was measured using charge coupled device (CCD) camera and image analysis software (LB-ADSA mode). The pH, surface tension and viscosity of the ink were obtained using 827 pH Metrohm meters, Tensiometer K100MK2 and Anton Paar MCR 300 Rheometer, correspondingly. The surface morphology and cross sectional view of printed PEDOT:PSS ink on the flexible PET substrate was determined through the use of a LEO; model 1455VP scanning electron microscope (SEM) and DME Dual Scope C320 model atomic force microscopy (AFM). Additionally, morphology and size of the PEDOT:PSS inks were observed under a transmission electron microscopy (TEM) (JEM-100CXII). Particle size of PEDOT:PSS was determined by dynamic light scattering (DLS) equipment (Malvern Instrument, UK; model ZEN 3600), which measured the random changes in the intensity of light scattered from an ink. Optical transmittance of the ink-jet printed PEDOT:PSS films were measured, within the wavelength range of 300 – 800 nm, by means of a UV/visible spectrometer (Agilent 8453). Sheet resistance of the ink-jet printed PEDOT:PSS films was measured by a WS-1 type four-probe apparatus.
2.3. Preparation of conductive inks
To prepare different ink-jet inks, four inks in which the percentage of PEDOT:PSS, deionized water, isopropyl alcohol and co-solvents (table 2) were same in their formulation but contained various types of co-solvents that have different structures, dipole moments and boiling points were prepared. The pH value of the inks was later set to  by a buffer solution to avoid any damage to printer head and cartridge. The prepared inks (Ink1 to Ink4) were ultra-sonicated for 10 min then filtered through a
by a buffer solution to avoid any damage to printer head and cartridge. The prepared inks (Ink1 to Ink4) were ultra-sonicated for 10 min then filtered through a  and
and  Sartorius Minisart filter in order to remove the particles, which had agglomerated during the ink formulation process.
Sartorius Minisart filter in order to remove the particles, which had agglomerated during the ink formulation process.
Table 2. The PEDOT:PSS inks formulations (wt%).
| Ink composition | Ink1 | Ink2 | Ink3 | Ink4 |
|---|---|---|---|---|
| PEDOT:PSS | 5 | 5 | 5 | 5 |
| DE-ionized water | 10 | 10 | 10 | 10 |
| Isopropanol | 2 | 2 | 2 | 2 |
| DEG | 1 | — | — | — |
| TRIEG | — | 1 | — | — |
| TETRAEG | — | — | 1 | — |
| PET | — | — | — | 1 |
2.4. Ink-jet printing of PEDOT:PSS inks
The ink-jet printing was undergone using an Epson Stylus Photo P50 printer sets to 1200 dpi. The printing process using the PEDOT:PSS ink formulation onto the flexible indium tin oxide coated PET was displayed in table 2. To remove any pollutant from prior print, the printer was flushed through with PEDOT:PSS ink. All substrates uncontaminated by the chemical solvents to remove surface contaminants and to provide a clean ITO surface to enhance the adhesion of ink onto its surface [41]. Therefore, ITO coated PET substrates were washed with deionized water, isopropyl alcohol, acetone, and methanol, respectively in an ultrasonic bath at room temperature, for 10 min separately. The methanol is an effective solvent for ITO cleaning, as it provides a higher surface energy [41]. Finally, the cleaned ITO coated PET dried at 80 °C for 1 h just before loading into the printing system. The prints were adjusted to 1 and 3 runs by a computer program to assess the differentiations in the electrical conductivity properties with variation in thickness of the printed film. Following initial drying of the printed film, the flexible ITO coated PET were heat-treated at optimum temperatures (130 °C) from previous study [40] for 10 min. Then, they were cooled down to the room temperature.
3. Results and discussion
3.1. Rheological behavior
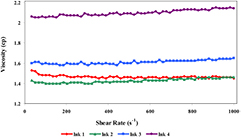
The viscosity of the prepared ink predominantly affects the rheological behaviors through the capillary nozzles of the printer. Figure 2 shows the variation of viscosities along with shear rates for the prepared water soluble inks on a logarithmic scale. It is apparent that the viscosity of the inks has remained almost unchanged by increasing the shear rate; demonstrating that the inks behave as a Newtonian fluid. Consequently, the type of co-solvents had no effect on rheological behavior of the PEDOT:PSS inks, and viscosity of the prepared inks was found to be in acceptable range at shear rate  [42]. However, the viscosity of the prepare inks increased from
[42]. However, the viscosity of the prepare inks increased from  to
to  at shear rate
at shear rate  by increasing the molecular weight of adding co-solvents.
by increasing the molecular weight of adding co-solvents.
Figure 2. Rheology behavior of PEDOT:PSS inkjet ink formulation at different shear rates.
Download figure:
Standard image High-resolution image3.2. Surface tension
Surface tension of the prepared inks plays important roles not only in wettability of the ink onto the substrate but also in decomposition of the ink into fine drops through the capillary tubes of the printer nozzle. Water, as the most important phase in the ink formulation, played the primary role in controlling surface tension. Surface tension of the prepared inks (table 3) was found to be in acceptable range  to
to  for commercial ink-jet inks [43].
for commercial ink-jet inks [43].
Table 3. Surface tension of PEDOT:PSS ink-jet inks formulations.
| Ink | Surface tension (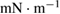 ) ) |
|---|---|
| 1 | 35.3 |
| 2 | 38.43 |
| 3 | 43.3 |
| 4 | 43.4 |
3.3. DLS of PEDOT:PSS inks
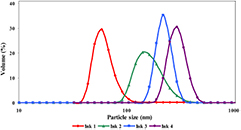
The size of the PEDOT:PSS particles in the prepared inks (Ink1-Ink4) were determined by dynamic light scattering (DLS). DLS results presented in figure 3 indicates, the particle size of PEDOT:PSS for all the prepared ink are on the acceptable range to use in ink-jet printing process. The DLS results show that, as the size of molecular structure of co-solvent increased, the particle size of PEDOT:PSS increased. These results prove that the type of co-solvents effect to the morphology of PEDOT:PSS chain.
Figure 3. Particle size distribution of PEDOT:PSS ink-jet ink formulations.
Download figure:
Standard image High-resolution imageThe PEDOT:PSS has a tertiary structure with a diameter of several tens nanometer, where hydrophobic PEDOT molecules aggregate to form physical cross-links between the PSS chains in water. Adding organic co-solvents in ink formulation may bring a screening effect between the positively charged PEDOT chains and negatively charged PSS chains and then reducing the coulomb interaction between them, which causes a phase separation between PEDOT and PSS (figure 4). Therefore, by increasing the molecular size of co-solvent the space between the PEDOT and PSS chains increased that cause increasing in the PEDOT: PSS particle size.
Figure 4. Particle structure of PEDOT:PSS inks in presence of co-solvent and without it.
Download figure:
Standard image High-resolution image3.4. Electrical properties
The conductivity of ten different locations on each ink-jet printed samples were measured and an average of the measured values were calculated and presented in table 4. The results in table 4 clearly show that the electrical of the PEDOT:PSS ink-jet printed depend noticeably on the types of co-solvents. An assessment of the conductivity values between the different co-solvents shows that the samples, which have higher dipole moment, boiling point, molecular weight as a co-solvent in the ink formulation have higher conductivity than the other samples. This is consistent with the difference in co-solvents formulated in ink-jet ink affects the printed film conductivity. In the PEDOT:PSS dispersion the polymer chains have shown in most occurrence to adapt a random coil conformation. A thin film is formed with grains containing of doped conjugated polymer coils, when the dispersion is printed onto a substrate [5, 6]. PSS chains are made up of several hundred monomer units, the polymer grains are distinguished by the PSS random coil with ionically attached PEDOT chains.
Table 4. Surface resistance of printed PEDOT:PSS on PET/ITO substrates.
| Ink | Surface resistance (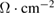 )/1 layer )/1 layer |
Surface resistance (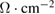 )/3 layers )/3 layers |
|---|---|---|
| 1 | 48.5 | 47.1 |
| 2 | 48.1 | 47.4 |
| 3 | 47.5 | 46.5 |
| 4 | 47.2 | 45.7 |
In polar and protic solvents, which have large dipole moment and can donate proton ( ) cause the removal of the negative charge PSS−chain (
) cause the removal of the negative charge PSS−chain ( ) to produce
) to produce  by effectively screening them from the positively charged hydrophobic PEDOT chains, which cause the phase separation between the chains. Consequently, as the dipoles of additive increased the coulomb interaction reduced increasingly, then because of loosely structure the PEDOT chains during film formation reoriented and leaving high conductivity of thin film on the surface of the substrate.
by effectively screening them from the positively charged hydrophobic PEDOT chains, which cause the phase separation between the chains. Consequently, as the dipoles of additive increased the coulomb interaction reduced increasingly, then because of loosely structure the PEDOT chains during film formation reoriented and leaving high conductivity of thin film on the surface of the substrate.
On the other hand, by increasing the molecular size of the co-solvent, the space between PEDOT and PSS chains increased. Then as the boiling point of co-solvent increased, the length of the time that co-solvent exist in between PEDOT and PSS chains raised (figure 4), thereby creating an opportunity for a favorable morphological rearrangement to a decreased resistance between dried particles, thus increasing the overall conductivity of the film. However, the boundaries between the dried particles in an ink-jet printed film contribute significantly to the overall resistivity of the film. Maximum conductivity is achieved by maximizing the contact between dried particles in PEDOT:PSS films. Therefore the conductivity enhancement is strongly dependent on the chemical structure, dipoles, and boiling point of co-solvent. By increasing dipole moments, and boiling point of co-solvent, the thin film conductivity on the surface of the substrate increase.
3.5. Effect of thickness on electrical and optical properties
Table 4 shows that the dependency of the electrical properties of the material to the number of runs performed with the PEDOT:PSS ink-jet inks. By increasing the number of printing runs, the conductivity of the printed film increase. This raising in conductivity might be due to increase the thickness and uniformity of the pattern by build-up a greater concentration of the conductive PEDOT:PSS on the substrate.
As the three runs of PEDOT:PSS ink-jet printed films had the optimum conductive properties, therefore, the optical properties of that optimum printed film were assessed by UV–VIS absorption spectroscopy. Figure 5 illustrates that the average transmittance of PEDOT:PSS ink-jet printed films (Ink1 to Ink4) on the visible light region slightly decreased by increasing not only the molecular weight of co-solvents but also the particle size distribution of PEDOT:PSS, for example the transparency of the Ink1, which formulated with DEG is  and for Ink 4 that consist PEG is
and for Ink 4 that consist PEG is  . It was assumed that, by increasing the particle size distribution of PEDOT:PSS the thickness of the printed film raised, hence the light reflection faintly increased. The ink-jet printed PEDOT:PSS films onto the flexible substrate exhibited have high transparency within the visible wavelength region and ideal for use in optoelectronic devices. Therefore, it is inferred that adding co-solvents to the PEDOT:PSS based ink-jet inks had a minor effect on optical properties of the PEDOT:PSS ink-jet printed film.
. It was assumed that, by increasing the particle size distribution of PEDOT:PSS the thickness of the printed film raised, hence the light reflection faintly increased. The ink-jet printed PEDOT:PSS films onto the flexible substrate exhibited have high transparency within the visible wavelength region and ideal for use in optoelectronic devices. Therefore, it is inferred that adding co-solvents to the PEDOT:PSS based ink-jet inks had a minor effect on optical properties of the PEDOT:PSS ink-jet printed film.
Figure 5. Transparency of PEDOT:PSS inkjet ink formulation in different wave length.
Download figure:
Standard image High-resolution image3.6. Morphology (AFM, SEM and TEM)
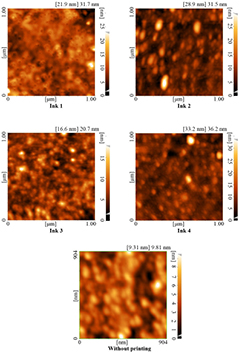
AFM topography images were obtained for different ink-jet printed films (Ink1 to Ink4), that contain various co-solvent in their formulation, to allow studies possible changes in morphology on a nanometer scale. The morphologies of the four samples were compared as measured by AFM topography images (figure 6). Therefore, the AFM images and results of the Ink1 to Ink4 printed films (table 5) confirmed that the roughness of PEDOT:PSS ink-jet printed films increased from 2.9 to 3.55 nm at root mean square (RMS) by increasing the molecular weight of co-solvent and PEDOT:PSS particle size distribution. Furthermore, the AFM images show a grain like structure of PEDOT:PSS film. Grain like structure was matched to the previous studies, which can be explained by the self-assembly and consequently increased inter-chain interaction occurring in the film form by ink-jet printing process [4, 44]. Therefore, adding co-solvent in the ink formulation effect on the roughness and morphology of the printed PEDO:PSS films.
Figure 6. 2D AFM images of printed different inkjet ink formulation of PEDOT:PSS.
Download figure:
Standard image High-resolution imageTable 5. The AFM results of printed different PEDOT:PSS inkjet inks formulation.
| Ink | Square (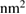 ) ) |
Size (nm) |
|---|---|---|
| 1 | 3 | 21.03 |
| 2 | 3.5 | 22.9 |
| 3 | 3.52 | 25.3 |
| 4 | 3.55 | 25.74 |
| Without printing | 1.35 | 7.36 |
SEM images of the ink-jet printed PEDOT:PSS films (Ink1–Ink4) are depicted in figure 7 demonstrates the addition co-solvent effect on the morphology of the ink-jet printed PEDOT:PSS. In SEM image the size of cauliflower like structures increased by increasing the boiling point and molecular weight of used co-solvent in ink-formulation.
Figure 7. SEM images of printed different inkjet ink formulation of PEDOT:PSS.
Download figure:
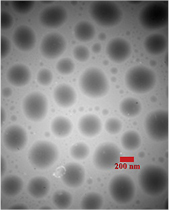
Standard image High-resolution imageTEM image of the optimum PEDOT:PSS ink is depicted in figure 8 that demonstrates micelles of PEDOT:PSS particles in the ink and clearly demonstrates the well dispersed particles with a minimal size variation (200 nm) in the ink.
Figure 8. TEM image of the optimal PEDOT:PSS ink-jet ink.
Download figure:
Standard image High-resolution image4. Conclusions
Studies on PEDOT:PSS ink-jet inks that blended with different organic co-solvents, which have different structure, dipole moment and boiling point, have shown that conductivity, transparency and roughness of ink-jet printed films can be increased by increasing the molecular weight, dipole moment and boiling point. The increasing in the electrical conductivity can be related to the screening effect of the (polar) solvent that improved the morphology of the film either by expanding the coiled PEDOT:PSS that increased conjugation length or made the polarons more delocalized and changes the charge transport properties. The best method of increasing electrical conductivity of PEDOT:PSS ink-jet printed films is mixing the PEDOT:PSS ink with polyethylene glycol. Therefore, adding co-solvent not only may played an important role in the physical properties of the PEDOT:PSS ink-jet inks but also in improving the conductivity and transparency of the printed films. On the other hand, by increasing the number of printing runs, the conductivity and thickness of the printed films increased. This raising might be due to uniformity of the pattern by build-up a greater concentration of the conductive PEDOT:PSS on the substrate.
Acknowledgments
This work was done as a research project in Institute for Color Science and Technology, by financial support of Iran Energy Efficiency Organization (IEEO).