Abstract
The use of plants in the biosynthesis of nanoparticles is a fast-growing technique and has gained much interest from researchers over the years. This study reports the utilization of leaf and calyx extracts of Plumbago auriculata for the biosynthesis of silver nanoparticles (AgNPs). The formation of AgNPs which was confirmed by the colour change in the plant extracts was characterized by UV-Vis spectrophotometric, TEM, SEM, EDX and FT-IR analyses. The water-soluble components of the extracts were responsible for the reduction of  ions. FT-IR analysis revealed the efficient capping and stabilization properties of these particles and the nature of the capping agent. The antibacterial properties of the biosynthesized AgNPs were evaluated against both gram-negative and gram-positive bacteria and the results obtained showed good antibacterial activity against Klebsiella pneumoniae. This was the first reported study for Plumbago auriculata and contributes to the environmentally friendly and cost-effective technique of the biosynthesis of nanoparticles for drug development.
ions. FT-IR analysis revealed the efficient capping and stabilization properties of these particles and the nature of the capping agent. The antibacterial properties of the biosynthesized AgNPs were evaluated against both gram-negative and gram-positive bacteria and the results obtained showed good antibacterial activity against Klebsiella pneumoniae. This was the first reported study for Plumbago auriculata and contributes to the environmentally friendly and cost-effective technique of the biosynthesis of nanoparticles for drug development.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is a fast-growing approach in the modern era. It has become one of the most active areas of research across the globe to explore the potential of synthesizing nanoparticles using different plants [1, 2]. Nanoparticles display characteristic properties in their distribution, size and morphology. Metals used to synthesize nanoparticles include copper, silver, gold, iron oxide, platinum, silica and nickel [3, 4]. Due to their unique properties and applications, metal nanoparticles have become quite popular over the years [5, 6]. Silver is the most commonly used metal for synthesizing nanoparticles. It is non-toxic to humans, active at low concentrations, and exhibits various applications in the following fields: antimicrobials and therapeutics, high sensitivity bimolecular diagnostics and detection as well as catalysis and micro-electronics [6–9]. Previous studies have reported that silver ions and silver based compounds are highly toxic to many microorganisms [10]. Due to the high antimicrobial properties of AgNPs they are used in numerous household products, the medical industry and in cosmetic and pharmaceutical products.
Synthesis of nanoparticles can be achieved through various methods. Chemical methods are the most popular approaches but they are often too expensive and can be toxic [3]. Biosynthesis has become a more widely used approach as it is cost effective, ecofriendly and has low toxicity. Biological methods include the use of enzymes, plant extracts and microorganisms [6, 11]. Biosynthesis using plants is the best method because it a safe option and plants are more easily available and widely distributed [12]. Factors affecting biosynthesis include temperature and pH with temperature variations in the synthesis process playing an important role in controlling the size and shape of the particles [4]. Aqueous (polar) media are reported to be more effective in biosynthesis than in non-polar media [4].
Plants have been used for centuries because of their medicinal properties. Currently, the biosynthesis of AgNPs with plant extracts is being exploited. This approach is more economical as it minimizes processing time and can be done on a small to large scale basis [3, 9]. Nanostructured systems are believed to potentiate the action of plant extracts, thereby improving their activity and reducing the required dose and possibly reducing adverse side effects [4, 13]. Plant-mediated biosynthesis is considered a widely acceptable technology for the rapid production of AgNPs but caution must be given to the type of extraction solvents used because of the hydrophobic nature of the capping agents that are used [5, 12].
Plants and plant metabolites exhibit strong antimicrobial properties and this has been confirmed by the number of scientific publications. The ethno-botanical knowledge and pharmacological studies on the aerial parts and roots of Plumbago auriculata (P. auriculata) suggest that the aerial parts and roots have a wide range of medicinal uses [14–16]. P. auriculata is a bushy evergreen shrub, common to South Africa and is also distributed in warm tropical regions in other parts of the world [17]. The various parts of the plant possess a wide range of phytochemicals. Plumbagin, the marker compound, shows various pharmacological activities including antimicrobial, anticancer, antifertility, antifungal, anti-inflammatory, antimalarial, antioxidant, hyperglycaemic and cardiotonic [18–20]. It was reported that the aqueous root extract of P. auriculata (=P. capensis Thunb.) effectively synthesizes AgNPs and can be effectively applied as an antifungal agent [21]. Other species in the genus Plumbago which are also reported to effectively synthesize nanoparticles include Plumbago indica L. and Plumbago zeylanica L. [1, 22]. The present study attempted to synthesize AgNPs with P. auriculata extracts and to characterize and evaluate the antimicrobial activity of the extracts.
2. Materials and method
2.1. Plant material and preparation of the extract
Plumbago auriculata was collected on the Westville Campus of the University of KwaZulu-Natal in Durban, South Africa. A voucher specimen has been deposited in the Ward Herbarium (Singh and Baijnath 1). Aqueous extracts of leaves and calyces were prepared using fresh samples (25 g) which were crushed in distilled water (100 ml) using a mortar and pestle. Samples were then filtered through Whatman no.1 filter paper and stored at  for 14 d.
for 14 d.
1 mM silver nitrate solution was prepared as follows: one molar silver nitrate stock solution was prepared by dissolving  (0.17 g) (BDH Chemicals Ltd England) in distilled water (100 ml). A
(0.17 g) (BDH Chemicals Ltd England) in distilled water (100 ml). A  solution was prepared by diluting 1 M solution (10 ml) in distilled water (90 ml). This solution was stored in a dark bottle for further use at room temperature. The concentrations of the extracts and
solution was prepared by diluting 1 M solution (10 ml) in distilled water (90 ml). This solution was stored in a dark bottle for further use at room temperature. The concentrations of the extracts and  were
were  and
and  , respectively.
, respectively.
2.2. Synthesis of AgNPs
Aqueous and methanolic extracts of leaf and calyx (5 ml each) were separately added to 1 mM  solution (45 ml) for reduction of
solution (45 ml) for reduction of  ions. Synthesis of AgNPs occurred at room temperature (
ions. Synthesis of AgNPs occurred at room temperature ( ) and at
) and at  by heating extracts in a water bath. The change in colour of the solution (this indicated the formation of the AgNPs) was observed and the time was recorded.
by heating extracts in a water bath. The change in colour of the solution (this indicated the formation of the AgNPs) was observed and the time was recorded.
2.3. Characterization of P. auriculata AgNPs
2.3.1. UV-Vis spectrophotometric analysis.
The reduction of pure  ions was monitored by using a UV-Vis spectrophotometer (Spectrostar Nano BMG, Germany). Distilled water was used as blank. The reaction medium was analyzed for its maximum absorption at a wavelength range of 220–600 nm and the corresponding peaks were recorded. The absorbance of the reaction medium was measured within 24 h.
ions was monitored by using a UV-Vis spectrophotometer (Spectrostar Nano BMG, Germany). Distilled water was used as blank. The reaction medium was analyzed for its maximum absorption at a wavelength range of 220–600 nm and the corresponding peaks were recorded. The absorbance of the reaction medium was measured within 24 h.
2.3.2. pH.
pH levels of the different extracts were measured before and after bioreduction using a pH meter (WS instruments, pH 50+, Italy) and calibrated at a two-point calibration between 4 and 7. The pH readings of water and silver were also recorded.
2.3.3. Scanning electron microscopy analysis and energy dispersive x-ray microanalysis.
Scanning electron microscopy (SEM) analysis was done using a Zeiss Ultra Plus field emission scanning electron microscope (FESEM, Germany) at 5 kV. Thin films of the sample were prepared on a carbon coated brass stub by adding a drop of the sample onto the coverslip mounted on the stub. The sample was allowed to air-dry for 10 min. Samples on the stubs were then sputter coated with gold for approximately 20 min. Shape and morphology of the nanoparticle clusters were identified. Energy dispersive x-ray (EDX) analysis was used to identify the composition of the synthesized AgNP and to confirm the presence of silver. EDX analysis was done using a Zeiss Ultra Plus FESEM with software Aztec (Oxford instruments, UK) at 5 kV.
2.3.4. Transmission electron microscopy analysis.
Transmission electron microscopy (TEM) analysis was performed to characterize the size and shape of the synthesized AgNPs. A drop of the nanoparticle solution was placed on a formvar coated copper grid and air dried for 10 min. Images were viewed using the Joel TEM 1010 (Japan) at 200 kV.
2.3.5. Fourier transform infrared analysis.
Fourier transform infrared (FTIR) measurements were carried out to identify the possible biomolecules responsible for reduction, capping and efficient stabilization of AgNPs and the local molecular environment of the capping agents on the nanoparticles [23]. FTIR of dried biomass after bioreduction was carried out by removing any free residue from the capping ligand. The residual solution of 50 ml after reaction was centrifuged at 5000 rpm for 15 min and the supernatant was decanted and the pellet formed was dried in an oven at  . The dried nanoparticle was analyzed in order to evaluate the bioreducing and capping functional groups of the silver nanoparticles. Infrared spectra of the crude extracts and their corresponding biosynthesized AgNPs were obtained on a Perkin Elmer Spectrum 100 FTIR (USA) with universal attenuated total reflectance (ATR) sampling accessory.
. The dried nanoparticle was analyzed in order to evaluate the bioreducing and capping functional groups of the silver nanoparticles. Infrared spectra of the crude extracts and their corresponding biosynthesized AgNPs were obtained on a Perkin Elmer Spectrum 100 FTIR (USA) with universal attenuated total reflectance (ATR) sampling accessory.
2.4. Antibacterial assay
Preliminary antibacterial screening of the biosynthesized AgNPs was carried out against 2 gram-positive bacteria (Staphylococcus aureus ATCC 25923 and Staphylococcus aureus Rosenbach ATCC BAA-1683 (methicillin resistant S. aureus, MRSA)) and 4 gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 31488, Escherichia coli ATCC 25922 and Salmonella typhimurium). The bacteria were grown overnight in Nutrient Broth (Biolab, South Africa) at  in a shaking incubator (100 rpm). The bacterial concentration was adjusted to 0.5 McFarland's Standard with sterile distilled water using a DEN-1B McFarland densitometer (Latvia). Mueller-Hinton agar (MHA) plates (Biolab, South Africa) were lawn inoculated with the prepared bacterial suspensions using a sterile throat swab and
in a shaking incubator (100 rpm). The bacterial concentration was adjusted to 0.5 McFarland's Standard with sterile distilled water using a DEN-1B McFarland densitometer (Latvia). Mueller-Hinton agar (MHA) plates (Biolab, South Africa) were lawn inoculated with the prepared bacterial suspensions using a sterile throat swab and  of the extracts were spotted onto the MHA plates. The plates were incubated at
of the extracts were spotted onto the MHA plates. The plates were incubated at  for 18 h and after incubations the plates were read to determine antibacterial activity which was denoted by clear zones in the area where the extracts were spotted. Based on the preliminary screening results, the minimum inhibition concentrations (MICs) were determined. The aqueous dispersions of AgNPs were serially diluted 2-fold with water ranging from
for 18 h and after incubations the plates were read to determine antibacterial activity which was denoted by clear zones in the area where the extracts were spotted. Based on the preliminary screening results, the minimum inhibition concentrations (MICs) were determined. The aqueous dispersions of AgNPs were serially diluted 2-fold with water ranging from  and
and  of each concentration was spotted on the lawn inoculated MHA plates and incubated at
of each concentration was spotted on the lawn inoculated MHA plates and incubated at  for 24 h. The crude extracts and
for 24 h. The crude extracts and  solution served as controls.
solution served as controls.
3. Results and discussions
A first experimental study on the biosynthesis of silver nanoparticles using leaf and calyx extracts of Plumbago auriculata was confirmed and compared to several existing studies in respective areas [4–7, 12, 17, 18, 20–31]. In the biosynthesis of nanoparticles, a colour change in the reaction mixture is the first step that indicates that nanoparticles have been synthesized [4]. In this study the reaction mixture displayed a wide range of colour changes resulting from the exposure to varying temperatures and extract media. When the reactions were placed in a water bath at  , the colour changed gradually within the first 30 min. After 2 h in the water bath at
, the colour changed gradually within the first 30 min. After 2 h in the water bath at  , the colour of the leaf extracts varied between yellowish brown and dark brown (figures 1(a)–(d)) and the colour of the calyx extracts ranged from light to dark brown (figures 1(e) and (h)). At room temperature the reactions took longer. After 1 h, a gradual change in colour was observed and after 2 h the extracts reached the end colour. The methanol extracts for both leaves and calyces resulted in a darker colour when exposed to
, the colour of the leaf extracts varied between yellowish brown and dark brown (figures 1(a)–(d)) and the colour of the calyx extracts ranged from light to dark brown (figures 1(e) and (h)). At room temperature the reactions took longer. After 1 h, a gradual change in colour was observed and after 2 h the extracts reached the end colour. The methanol extracts for both leaves and calyces resulted in a darker colour when exposed to  (figures 1(b) and (g)). The change in colour visually observed in this study was similar to previous studies [17, 21, 26, 31, 32]. However, it was reported that with the formation of AgNPs using Plumbago capensis root extract, the colour changed from clear to reddish orange [20]. The change in colour after bioreduction is due to the excitation of the surface plasmon resonance (SPR) in AgNPs [7], thereby confirming the bioreduction of
(figures 1(b) and (g)). The change in colour visually observed in this study was similar to previous studies [17, 21, 26, 31, 32]. However, it was reported that with the formation of AgNPs using Plumbago capensis root extract, the colour changed from clear to reddish orange [20]. The change in colour after bioreduction is due to the excitation of the surface plasmon resonance (SPR) in AgNPs [7], thereby confirming the bioreduction of  ions in the reaction between the plant extract and
ions in the reaction between the plant extract and  [3, 12, 31].
[3, 12, 31].
Figure 1. Colour changes observed in the biosynthesis of AgNPs at two different temperatures of the different extracts: (a) yellowish brown of leaf methanolic extract  ; (b) dark brown of leaf methanolic extract
; (b) dark brown of leaf methanolic extract  ; (c) yellowish brown of leaf water extract
; (c) yellowish brown of leaf water extract  ; (d) yellowish brown of leaf water extract
; (d) yellowish brown of leaf water extract  ; (e) yellowish brown of calyx methanolic extract
; (e) yellowish brown of calyx methanolic extract  ; (f) yellowish brown calyx water extract
; (f) yellowish brown calyx water extract  ; (g) dark brown of calyx methanolic extract
; (g) dark brown of calyx methanolic extract  , and (h) yellowish brown of calyx water extract
, and (h) yellowish brown of calyx water extract  .
.
Download figure:
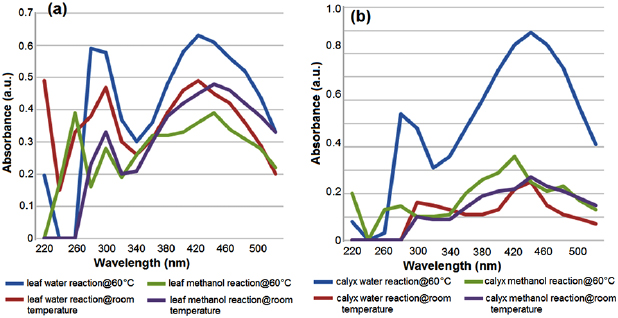
Standard image High-resolution imageEach metallic nanoparticle has its own characteristic absorption pattern [13, 23, 33]. UV-Vis spectroscopy is a valuable technique to establish the formation and stability of metal nanoparticles. It is well documented that the optical absorption spectra of metal nanoparticles are dominated by SPR that shift to longer wavelengths with increasing particle size [12]. In AgNPs, the appearance of SPR peaks at 446 nm provides a convenient spectroscopic signature for the formation of AgNPs. Absorption spectra of AgNPs formed have an absorption maximum in the range 440–460 nm. The UV-Vis spectra recorded implied that most rapid bioreduction was achieved at  for the leaf and calyx water extracts. This was denoted by broadening of the peaks that was indicative of the formation of large polydispersed nanoparticles due to slow reduction rates [5]. It was reported that maximum absorption rate for P. capensis aqueous root extract was at 420 nm [21]. Reduction of
for the leaf and calyx water extracts. This was denoted by broadening of the peaks that was indicative of the formation of large polydispersed nanoparticles due to slow reduction rates [5]. It was reported that maximum absorption rate for P. capensis aqueous root extract was at 420 nm [21]. Reduction of  ions in this study was confirmed by the UV-Vis spectroscopy. The UV-Vis spectrum of AgNPs (figure 2) was recorded from the reaction mediums, 24 h after synthesis. The samples showed similar behavior with maximum absorption peaks ranging between 420–460 nm. Reduction of silver ions by the extracts was evident by the UV-Vis spectroscopy. UV-Vis analysis was in accordance with earlier studies using aqueous leaf extract of Xanthium strumarium L. and carbohydrates of Chlorella vulgaris [17, 21, 34].
ions in this study was confirmed by the UV-Vis spectroscopy. The UV-Vis spectrum of AgNPs (figure 2) was recorded from the reaction mediums, 24 h after synthesis. The samples showed similar behavior with maximum absorption peaks ranging between 420–460 nm. Reduction of silver ions by the extracts was evident by the UV-Vis spectroscopy. UV-Vis analysis was in accordance with earlier studies using aqueous leaf extract of Xanthium strumarium L. and carbohydrates of Chlorella vulgaris [17, 21, 34].
Figure 2. UV-Vis absorption spectra of aqueous silver nitrate with 1 ml P. auriculata (a) leaf and (b) calyx extracts 24 h after synthesis.
Download figure:
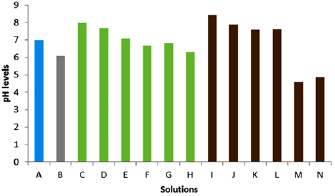
Standard image High-resolution imageThe pH levels for water (pH 7) and silver nitrate solution (pH 6) was fairly neutral (columns (A) and (B) in figure 3). The calyx crude extracts were slightly more alkaline prior to bioreduction (pH 7.9 and pH 7.6) (columns (C) and (D) in figure 3) but after bioreduction of  ions pH levels remained fairly neutral (columns (E)–(H) in figure 3). The aqueous leaf extracts showed high levels of alkalinity before bioreduction (pH 8.4 and pH 7.8) (columns (I) and (J) in figure 3) and after bioreduction (pH 7.6 and pH 7.61) (columns (K) and (L) in figure 3) whilst the methanol solutions were more acidic after bioreduction (pH 4.6 and pH 4.8) (columns (M) and (N) in figure 3). As previously reported, aqueous mediums are more effective in biosynthesis because pH affects the production and stability of the nanoparticles [4, 19].
ions pH levels remained fairly neutral (columns (E)–(H) in figure 3). The aqueous leaf extracts showed high levels of alkalinity before bioreduction (pH 8.4 and pH 7.8) (columns (I) and (J) in figure 3) and after bioreduction (pH 7.6 and pH 7.61) (columns (K) and (L) in figure 3) whilst the methanol solutions were more acidic after bioreduction (pH 4.6 and pH 4.8) (columns (M) and (N) in figure 3). As previously reported, aqueous mediums are more effective in biosynthesis because pH affects the production and stability of the nanoparticles [4, 19].
Figure 3. pH levels of the different solutions before and after synthesis of AgNPs, (A) water; (B)  ; (C) calyx crude water extract; (D) calyx crude methanol extract; (E) AgNPs synthesized using calyx water extract
; (C) calyx crude water extract; (D) calyx crude methanol extract; (E) AgNPs synthesized using calyx water extract  ; (F) AgNPs synthesized using calyx water extract
; (F) AgNPs synthesized using calyx water extract  ; (G) AgNPs synthesized using calyx methanol extract
; (G) AgNPs synthesized using calyx methanol extract  ; (H) AgNPs synthesized using calyx methanol extract
; (H) AgNPs synthesized using calyx methanol extract  ; (I) leaf crude water extract; (J) leaf crude methanol extract; (K) AgNPs synthesized using leaf water extract
; (I) leaf crude water extract; (J) leaf crude methanol extract; (K) AgNPs synthesized using leaf water extract  ; (L) AgNPs synthesized using leaf water extract
; (L) AgNPs synthesized using leaf water extract  ; (M) AgNPs synthesized using leaf methanol extract
; (M) AgNPs synthesized using leaf methanol extract  ; (N) AgNPs synthesized using leaf methanol extract
; (N) AgNPs synthesized using leaf methanol extract  .
.
Download figure:
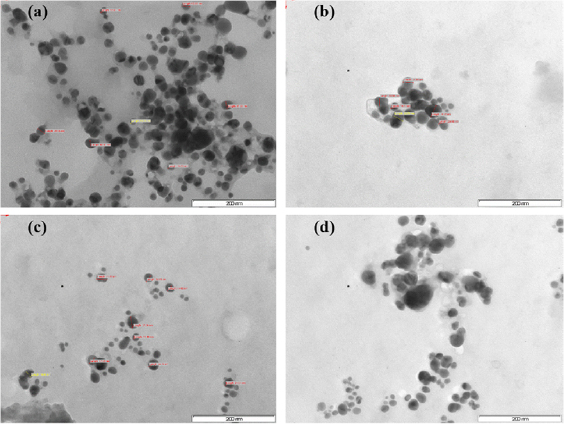
Standard image High-resolution imageElectron micrographs showed particles of varied shapes and sizes. Size and shape determine the efficacy of the particles. The smaller the particle sizes the greater are their properties [19]. The TEM images showed relatively spherical to oblong shape nanoparticles formed with diameters in the range of 15–30 nm. Analysis of the TEM images revealed that at room temperature spherical to oblong AgNPs ranging in size from 15.22 nm to 26.5 nm of both leaves and calyces were formed (figures 4(a) and (b)) whilst at  particles appear to be spherical but become aggregated with rising temperature. The size range was 18.33 nm to 29.48 nm for the leaves and calyces, respectively (figures 4(c) and (d)). The AgNPs were synthesized at
particles appear to be spherical but become aggregated with rising temperature. The size range was 18.33 nm to 29.48 nm for the leaves and calyces, respectively (figures 4(c) and (d)). The AgNPs were synthesized at  appeared slightly larger than the AgNPs synthesized at room temperature and were predominantly spherical to oblong in shape. Similar phenomena were also reported [1, 6, 21, 32, 35]. It is also reported that the shape of metal nanoparticles can considerably change their optical and electronic properties [24, 30, 31, 36]. The nanoparticles observed in this study tend to aggregate with increased temperature. This is a result of a decreased binding force between AgNPs and the capping molecules that tend to decrease with increasing temperatures [32]. Earlier reports also stated that higher temperatures favour smaller size particles in higher yields and uniform size distribution [19]. However, the findings of this study were not in favour of the larger particle size reported for Eriobotrya japonica [19]. The TEM images showed that the high density AgNPs were synthesized by the P. auriculata leaf and calyx extracts and this further confirmed the development of silver nanostructures. These outcomes are in the range of other reports [1, 9, 21, 31, 35].
appeared slightly larger than the AgNPs synthesized at room temperature and were predominantly spherical to oblong in shape. Similar phenomena were also reported [1, 6, 21, 32, 35]. It is also reported that the shape of metal nanoparticles can considerably change their optical and electronic properties [24, 30, 31, 36]. The nanoparticles observed in this study tend to aggregate with increased temperature. This is a result of a decreased binding force between AgNPs and the capping molecules that tend to decrease with increasing temperatures [32]. Earlier reports also stated that higher temperatures favour smaller size particles in higher yields and uniform size distribution [19]. However, the findings of this study were not in favour of the larger particle size reported for Eriobotrya japonica [19]. The TEM images showed that the high density AgNPs were synthesized by the P. auriculata leaf and calyx extracts and this further confirmed the development of silver nanostructures. These outcomes are in the range of other reports [1, 9, 21, 31, 35].
Figure 4. TEM micrographs of AgNPs synthesized at different temperatures using leaf and calyx extracts of P.auriculata: (a) synthesis with leaf extract  ; (b) synthesis with calyx extract
; (b) synthesis with calyx extract  ; (c) synthesis with leaf extract
; (c) synthesis with leaf extract  , and (d) synthesis with calyx extract
, and (d) synthesis with calyx extract  . 200 nm scale bar at 200 kV.
. 200 nm scale bar at 200 kV.
Download figure:
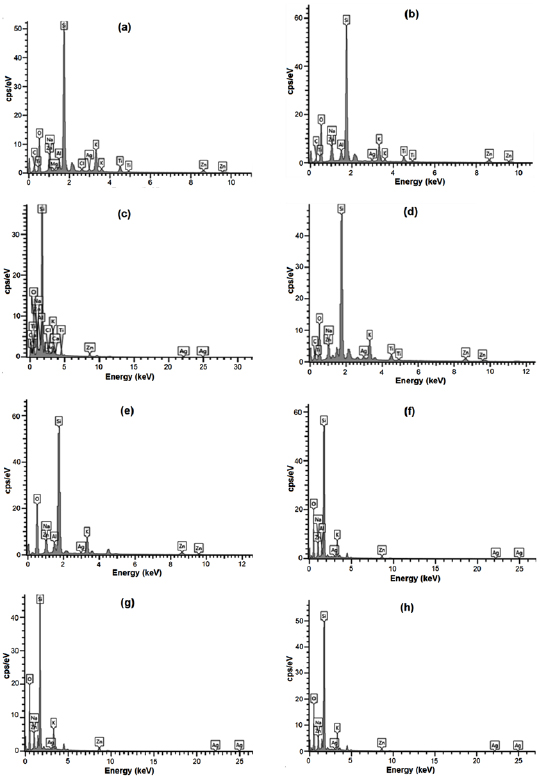
Standard image High-resolution imageEDX analysis further confirmed the presence of silver metal. From the EDX spectra (figure 5), it can be seen that AgNPs reduced by P. auriculata have a weight percentage of silver as follows: 1.7%, 8.5%, 0.65%, 2.56%, 0.58%, 0.82%, 0.83% and 0.85% (figures 5(a)–(h), respectively). From the EDX spectra of the AgNPs that was recorded, it can be deduced that AgNPs reduced by P. auriculata have varying weight percentages of silver. Previous studies have reported a much higher weight percentage of silver (16.41%) from synthesized AgNPs in comparison to the results of this study [2, 11, 26]. However, this could be a result of a larger quantity of  prepared and used in the biosynthesis process.
prepared and used in the biosynthesis process.
Figure 5. EDX spectra recorded after formation of AgNPs with different x-ray emission peaks labeled for the different extracts of P. auriculata: (a) leaf methanol  ; (b) leaf methanol
; (b) leaf methanol  ; (c) leaf water
; (c) leaf water  ; (d) leaf water
; (d) leaf water  ; (e) calyx methanol
; (e) calyx methanol  ; (f) calyx methanol
; (f) calyx methanol  ; (g) calyx water
; (g) calyx water  ; (h) calyx water
; (h) calyx water  .
.
Download figure:
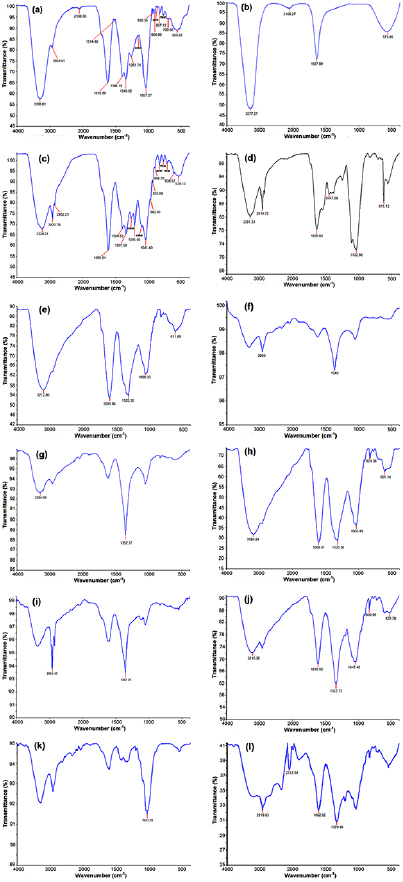
Standard image High-resolution imageFTIR analysis was used to characterize the extracts and the resulting AgNPs. FTIR absorption spectra of methanol and water-soluble extracts before and after reduction of  ions are shown in figure 6. Absorbance bands in figures 6(a)–(d) (before reduction) observed in the region
ions are shown in figure 6. Absorbance bands in figures 6(a)–(d) (before reduction) observed in the region  and in figures 6(e)–(l) (after reduction) are approximately 1037, 1041,1083, 1100, 1158, 1205, 1258, 1261, 1343, 1396, 1447, 1514, 1609, 1619, 1637, 2108, 2852, 2920, 2934, 3224, 3277 and
and in figures 6(e)–(l) (after reduction) are approximately 1037, 1041,1083, 1100, 1158, 1205, 1258, 1261, 1343, 1396, 1447, 1514, 1609, 1619, 1637, 2108, 2852, 2920, 2934, 3224, 3277 and  . These absorbance bands are known to be associated with stretching vibrations for –C–O–C–, ether linkages, –C–O–, terminal methyls, –C–C– groups or from aromatic rings and alkyne bonds, respectively, and N–H, primary and secondary amines and O–H groups. These bands denote stretching vibrational bands responsible for compounds like terpenoids and flavonoids and revealed the capping of AgNPs. The absorption peak at
. These absorbance bands are known to be associated with stretching vibrations for –C–O–C–, ether linkages, –C–O–, terminal methyls, –C–C– groups or from aromatic rings and alkyne bonds, respectively, and N–H, primary and secondary amines and O–H groups. These bands denote stretching vibrational bands responsible for compounds like terpenoids and flavonoids and revealed the capping of AgNPs. The absorption peak at  is close to that reported for proteins, suggesting that proteins are interacting with biosynthesized AgNPs. Absorption peaks occurring between
is close to that reported for proteins, suggesting that proteins are interacting with biosynthesized AgNPs. Absorption peaks occurring between  denote alcohols (O–H bands) possibly arising from proteins and carbohydrates present in the sample which is in agreement with the value reported in the literature [4]. The main peaks (–C=O and O–H bands) present in the extracts are also present in the AgNPs, inferring the attachment of biomolecules which are present in the extract to nanoparticles. It has already been reported that the hydroxyl groups act as reducing agents and the carboxyl group promotes size and shape of the nanoparticles [12] The data presented in this study indicate the involvement of –C=O and –OH functional groups in the reduction and stabilization of AgNPs which was in accordance to previous studies [14, 30, 35].
denote alcohols (O–H bands) possibly arising from proteins and carbohydrates present in the sample which is in agreement with the value reported in the literature [4]. The main peaks (–C=O and O–H bands) present in the extracts are also present in the AgNPs, inferring the attachment of biomolecules which are present in the extract to nanoparticles. It has already been reported that the hydroxyl groups act as reducing agents and the carboxyl group promotes size and shape of the nanoparticles [12] The data presented in this study indicate the involvement of –C=O and –OH functional groups in the reduction and stabilization of AgNPs which was in accordance to previous studies [14, 30, 35].
Figure 6. FTIR spectra of crude plant extracts and biosynthesized AgNPs of P. auriculata (a) crude leaf methanol extract; (b) crude leaf water extract; (c) crude calyx methanol extract; (d) crude calyx water extract; (e) synthesis with leaf methanol extract  ; (f) synthesis with leaf water extract
; (f) synthesis with leaf water extract  ; (g) synthesis with leaf methanol extract
; (g) synthesis with leaf methanol extract  ; (h) synthesis with leaf water extract
; (h) synthesis with leaf water extract  ; (i) synthesis with calyx methanol extract
; (i) synthesis with calyx methanol extract  ; (j) synthesis with calyx water extract
; (j) synthesis with calyx water extract  ; (k) synthesis with calyx water extract
; (k) synthesis with calyx water extract  ; (l) synthesis with calyx methanol extract
; (l) synthesis with calyx methanol extract  .
.
Download figure:
Standard image High-resolution imageIn this study the MIC for gram-negative and gram-positive bacteria is presented in table 1. The biosynthesized AgNPs showed antibacterial activity on all microorganisms tested. The crude extracts displayed antibacterial activity at  against all the bacteria tested. The synthesized nanoparticles and the
against all the bacteria tested. The synthesized nanoparticles and the  were active against all the bacteria tested. For E. coli,
were active against all the bacteria tested. For E. coli,  had higher activity than the AgNPs for all extracts with the exception of nanoparticles synthesized from the calyx extract at
had higher activity than the AgNPs for all extracts with the exception of nanoparticles synthesized from the calyx extract at  (MIC of
(MIC of  ). For S. aureus, the aqueous leaf extract AgNPs at
). For S. aureus, the aqueous leaf extract AgNPs at  displayed the highest activities whilst for K. pneumoniae the AgNPs showed higher or similar activity as compared with
displayed the highest activities whilst for K. pneumoniae the AgNPs showed higher or similar activity as compared with  . AgNPs which synthesized with aqueous calyx extract at
. AgNPs which synthesized with aqueous calyx extract at  showed the most activity against K. pneumoniae with an MIC of
showed the most activity against K. pneumoniae with an MIC of  . For S. typhimurium, the AgNPs displayed a higher activity than the
. For S. typhimurium, the AgNPs displayed a higher activity than the  with both samples with a MIC of
with both samples with a MIC of  . Results obtained in previous studies support the antibacterial potential of AgNPs obtained in this study [7, 8, 25, 31, 34, 35].
. Results obtained in previous studies support the antibacterial potential of AgNPs obtained in this study [7, 8, 25, 31, 34, 35].
Table 1. Minimum inhibitory concentration (MIC) of biosynthesized silver nanoparticles.
| Microorganism | Sample | MIC  |
||
|---|---|---|---|---|
| Extract |  |
AgNPs | ||
| Escherichia coli | 4 | 17000 | 15.63 | 15.63 |
| 5 | 17000 | 15.63 | 125 | |
| 6 | 17000 | 15.63 | 250 | |
| 7 | 17000 | 15.63 | 62.5 | |
| 8 | 17000 | 15.63 | 1.25 | |
| Staphylococcus aureus | 3 | 17000 | 10000 | 250 |
| 4 | 17000 | 10000 | 125 | |
| Klebsiella pneumoniae | 1 | 17000 | 500 | 250 |
| 2 | 17000 | 500 | 500 | |
| 3 | 17000 | 500 | 125 | |
| 4 | 17000 | 500 | 125 | |
| 5 | 17000 | 500 | 125 | |
| 6 | 17000 | 500 | 62.5 | |
| 7 | 17000 | 500 | 500 | |
| 8 | 17000 | 500 | 125 | |
| Salmonella typhimurium | 7 | 17000 | 10000 | 250 |
| 8 | 17000 | 10000 | 250 | |
Sample ID: 1-leaves methanol extract  ; 2-leaves methanol extract
; 2-leaves methanol extract  ; 3-leaves water extract
; 3-leaves water extract  ; 4-leaves water extract
; 4-leaves water extract  ; 5-calyx water extract
; 5-calyx water extract  ; 6-calyx water extract
; 6-calyx water extract  ; 7-calyx methanol extract
; 7-calyx methanol extract  ; 8-calyx methanol extract
; 8-calyx methanol extract  .
.
AgNPs prepared from leaf and calyx extracts showed maximum activity against K. pneumoniae. Results presented in this study are consistent with previous reports stating that gram positive bacteria are less prone to the antibacterial activity of silver salts [8, 16, 18]. In this study the biosynthesized AgNPs were found to have higher antibacterial activities as compared to the crude extracts. This is in accordance with previous studies that have reported better antibacterial activity of AgNPs than the crude extracts [32, 35]. As in previous studies, this study reveals that plant extracts and silver in their nano form are biologically active and can be used as antibacterial agents in drug development [12, 26]. This is the first reported study for P. auriculata and confirms the biosynthesized nanoparticles to be biologically active.
4. Conclusion
AgNPs were successfully synthesized using P. auriculata leaf and calyx extracts. The synthesis of AgNPs was confirmed by the change in colour upon addition of the  solution to the extracts. Characterization by UV-Vis, TEM, SEM and EDX analysis further confirmed the reduction of
solution to the extracts. Characterization by UV-Vis, TEM, SEM and EDX analysis further confirmed the reduction of  ions. FTIR spectra revealed that the –OH and –C=O groups present in the biomolecules were responsible for the stabilization and reduction of the AgNPs. AgNPs had desirable antibacterial properties against both gram-negative and gram-positive bacteria in comparison to the crude plant extracts and the
ions. FTIR spectra revealed that the –OH and –C=O groups present in the biomolecules were responsible for the stabilization and reduction of the AgNPs. AgNPs had desirable antibacterial properties against both gram-negative and gram-positive bacteria in comparison to the crude plant extracts and the  solution and, hence, has great potential in the preparation of drugs. Biosynthesis of AgNPs using P. auriculata extracts provides an environmentally friendly and cost-effective option in comparison to chemical and physical synthesizing techniques. The results obtained in this study were the first to be reported for this species, thus adding to the current knowledge of biosynthesized metal nanoparticles.
solution and, hence, has great potential in the preparation of drugs. Biosynthesis of AgNPs using P. auriculata extracts provides an environmentally friendly and cost-effective option in comparison to chemical and physical synthesizing techniques. The results obtained in this study were the first to be reported for this species, thus adding to the current knowledge of biosynthesized metal nanoparticles.
Acknowledgments
The authors wish to thank the University of Kwa-Zulu Natal, Westville Campus for providing the resources needed to conduct the research, The National Research Foundation (NRF) for funding this research and The Chemistry and Discipline of Pharmaceutical Sciences of the University of Kwa-Zulu Natal, Westville, for providing the laboratory facilities to conduct certain aspects of the research, The Microscopy and Microanalysis unit for facilities. The following people are thanked for their assistance Dr. Olusola Bodede for chemical guidance and input, Mr Ashlin Munsamy for technical assistance and Dr Karmini Muduray for assistance with spectrophotometry.