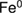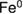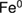Abstract
Zero-valent iron nanoparticles ( nanoparticles) with particle sizes in range 2–5 nm were synthesized using guava leaf extract from the reduction of
nanoparticles) with particle sizes in range 2–5 nm were synthesized using guava leaf extract from the reduction of  soluble precursor. The most effective solvent for the extraction of polyphenolic compounds from guava leaf was identified to contain 70% hydroethanolic solution. It was also found that this extract gives high reduction potential and a strong chelating ability for the preparation of
soluble precursor. The most effective solvent for the extraction of polyphenolic compounds from guava leaf was identified to contain 70% hydroethanolic solution. It was also found that this extract gives high reduction potential and a strong chelating ability for the preparation of  nanoparticles from soluble
nanoparticles from soluble  in basic aqueous solution at room temperature. At pH 8, the deprotonation of the polyphenolic groups with terminal –OH provides the strong binding sites to form a stable complex with
in basic aqueous solution at room temperature. At pH 8, the deprotonation of the polyphenolic groups with terminal –OH provides the strong binding sites to form a stable complex with  and can take part in the redox reduction. The formation and growth of
and can take part in the redox reduction. The formation and growth of  nanoparticles were monitored by UV-visible absorption spectroscopy and transmission electron microscopy. The achieved high dispersion and narrow size distribution of
nanoparticles were monitored by UV-visible absorption spectroscopy and transmission electron microscopy. The achieved high dispersion and narrow size distribution of  nanoparticles are attributed to the ability of tannins which are the major components of the polyphenolic groups to form strong complex with
nanoparticles are attributed to the ability of tannins which are the major components of the polyphenolic groups to form strong complex with  and stabilize
and stabilize  nanoparticles from aggregation. Methylene blue degradation in an aqueous solution was used as a model for study catalytic reduction performance of the synthesized tannin-stabilized
nanoparticles from aggregation. Methylene blue degradation in an aqueous solution was used as a model for study catalytic reduction performance of the synthesized tannin-stabilized  nanoparticles in colloidal form.
nanoparticles in colloidal form.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Zero-valent iron nanoparticles ( nanoparticles) have attracted considerable recent interest in environmental applications [1]. Due to their high surface area,
nanoparticles) have attracted considerable recent interest in environmental applications [1]. Due to their high surface area,  nanoparticles can efficiently remove many robust toxic contaminants in a short time. At present, zero-valent iron nanoparticles are the most commonly used nanomaterials for soil and groundwater remediation targeting mainly chlorinated organic contaminants and inorganic pollutants from aqueous solution [2, 3].
nanoparticles can efficiently remove many robust toxic contaminants in a short time. At present, zero-valent iron nanoparticles are the most commonly used nanomaterials for soil and groundwater remediation targeting mainly chlorinated organic contaminants and inorganic pollutants from aqueous solution [2, 3].
The most common approach for the synthesis of  nanoparticles is via chemical reduction of soluble iron ions [4]. However, this method requires the use of strong and toxic reducing agents such as sodium borohydride and hydrazine, which create considerably impacts to the environment. A reported green method for the synthesis of metal nanoparticles is via biosynthesis based on using plant extract with high antioxidant power [5]. Typically, this green synthesis of metal nanoparticles also involves a stabilization of small size of
nanoparticles is via chemical reduction of soluble iron ions [4]. However, this method requires the use of strong and toxic reducing agents such as sodium borohydride and hydrazine, which create considerably impacts to the environment. A reported green method for the synthesis of metal nanoparticles is via biosynthesis based on using plant extract with high antioxidant power [5]. Typically, this green synthesis of metal nanoparticles also involves a stabilization of small size of  by its bulky steric organic groups against its aggregation. Green synthesis provides advancement over chemical and physical methods as it is cost-effective, environment-friendly, easily scaled up for large-scale synthesis and there is no need to use high pressure, energy, temperature and toxic chemicals [6].
by its bulky steric organic groups against its aggregation. Green synthesis provides advancement over chemical and physical methods as it is cost-effective, environment-friendly, easily scaled up for large-scale synthesis and there is no need to use high pressure, energy, temperature and toxic chemicals [6].
There have been many reports on the green synthesis of novel metal nanoparticles such as Ag, Au and Pt nanoparticles using plant extracts [7, 8]. However, the green synthesis of  nanoparticles is scarcely reported [9, 10]. This is challenging to achieve due to the low standard reduction potential of Fe(II/III) ions and the potential rapid aggregation of particles due to their chemical and magnetic interactions. The source of plant extract, its concentration, the concentration of metal precursor, pH, reaction temperature and reaction time are known to influence on the characteristics of metal nanoparticles synthesised [11]. Tea extract has been reported to be active for the synthesis Fe nanoparticles [12]. Hoag et al [13] synthesized Fe nanoparticles using green tea extract and 0.1 M
nanoparticles is scarcely reported [9, 10]. This is challenging to achieve due to the low standard reduction potential of Fe(II/III) ions and the potential rapid aggregation of particles due to their chemical and magnetic interactions. The source of plant extract, its concentration, the concentration of metal precursor, pH, reaction temperature and reaction time are known to influence on the characteristics of metal nanoparticles synthesised [11]. Tea extract has been reported to be active for the synthesis Fe nanoparticles [12]. Hoag et al [13] synthesized Fe nanoparticles using green tea extract and 0.1 M  . The spherical Fe nanoparticles with particle size diameter in range 5–10 nm were obtained in a few minutes synthetic procedure at room temperature. Shahwan et al [14] also prepared Fe nanoparticles using green tea extract but using
. The spherical Fe nanoparticles with particle size diameter in range 5–10 nm were obtained in a few minutes synthetic procedure at room temperature. Shahwan et al [14] also prepared Fe nanoparticles using green tea extract but using  as a chemical precursor and the reduction performed under pH 6. The obtained nanoparticles demonstrated irregular clusters with some dispersion of discrete particle (40–60 nm). A similar particle size of Fe nanoparticles was obtained from the synthesis using oolong tea [15]. However, the particle size and morphology of nanoparticles could be changed by altering the concentration of extract as well as the use of different iron salts. Extracts from other plants have also been reported for the synthesis of Fe nanoparticles, such as eucalyptus [16], neem [17], grape [18] and clove [19] etc. Although these extracts have the strong reduction potentials to produce
as a chemical precursor and the reduction performed under pH 6. The obtained nanoparticles demonstrated irregular clusters with some dispersion of discrete particle (40–60 nm). A similar particle size of Fe nanoparticles was obtained from the synthesis using oolong tea [15]. However, the particle size and morphology of nanoparticles could be changed by altering the concentration of extract as well as the use of different iron salts. Extracts from other plants have also been reported for the synthesis of Fe nanoparticles, such as eucalyptus [16], neem [17], grape [18] and clove [19] etc. Although these extracts have the strong reduction potentials to produce  nanoparticles, their ability to protect freshly formed Fe nanoparticles from aggregation is not reported nor optimized.
nanoparticles, their ability to protect freshly formed Fe nanoparticles from aggregation is not reported nor optimized.
Guava leaves (Psidium guajava L) commonly used as a traditional medicine, and can be made available in all seasons and everywhere. Polyphenolic compounds as flavonoids and tannins are the main components in guava leaf with high antioxidant activities [20]. There are few reports on the synthesis of Ag nanoparticles using guava leaf extract [21]. However, there has not yet had any documentation on the green synthesis of  nanoparticles using guava leaf extract. In this work, we report for the first time the synthesis of high dispersion and narrowed size distribution of
nanoparticles using guava leaf extract. In this work, we report for the first time the synthesis of high dispersion and narrowed size distribution of  nanoparticles using guava leaf extract in basic solution. The catalytic performance of
nanoparticles using guava leaf extract in basic solution. The catalytic performance of  nanoparticles synthesized by green guava leaf extract for the removal of toxic contaminants in aqueous solution is also evaluated using methylene blue dye as a model.
nanoparticles synthesized by green guava leaf extract for the removal of toxic contaminants in aqueous solution is also evaluated using methylene blue dye as a model.
2. Experimental
2.1. Preparation of guava leaf extract
Guava leaves were collected from Prachinburi province, Thailand. The obtained samples were washed with tap water before sunlight drying during a day. Then, the dried samples were heated in an oven at  for an hour before grinding into a fine powder. An extraction was prepared via pre-mixing 4.0 g of the powder and 100.0 ml of extraction solvent, and then heated at
for an hour before grinding into a fine powder. An extraction was prepared via pre-mixing 4.0 g of the powder and 100.0 ml of extraction solvent, and then heated at  with stirring for 15 min. The extract was filtered using Whatman's no 1 filter paper. The powder was repeatedly treated with the solvent until a clear extract was obtained. After rotary evaporation, the crude extract was kept in the refrigerator. The abilities of solvents namely water, absolute ethanol and hydroethanolic solvents on the extraction yields were evaluated. The stock solution of the extract was prepared by dissolving 5.0 g of crude extract in 1.0 l of distilled water.
with stirring for 15 min. The extract was filtered using Whatman's no 1 filter paper. The powder was repeatedly treated with the solvent until a clear extract was obtained. After rotary evaporation, the crude extract was kept in the refrigerator. The abilities of solvents namely water, absolute ethanol and hydroethanolic solvents on the extraction yields were evaluated. The stock solution of the extract was prepared by dissolving 5.0 g of crude extract in 1.0 l of distilled water.
2.2. Estimation of total phenolic contents
The total phenolic content was estimated using Folin-Ciocalteu method [22]. Typically, 0.2 ml of the stock solution was diluted with 2.0 ml of distilled water and added with 0.2 ml of Folin-Ciocalteu's reagent, which was allowed to stand for 5 min. Then, 2.0 ml of 7% w/v of  solution was added, and the mixture was allowed to stand for 90 min at room temperature. The concentration of the phenolic content was measured using a UV-visible spectrophotometer at the wavelength of 765 nm. A standard calibration curve was prepared by plotting UV–vis absorbance against the concentration of gallic acid
solution was added, and the mixture was allowed to stand for 90 min at room temperature. The concentration of the phenolic content was measured using a UV-visible spectrophotometer at the wavelength of 765 nm. A standard calibration curve was prepared by plotting UV–vis absorbance against the concentration of gallic acid  . The total phenolic content of the extract was expressed as gallic acid equivalent (mg of gallic acid/g crude extract).
. The total phenolic content of the extract was expressed as gallic acid equivalent (mg of gallic acid/g crude extract).
2.3. Estimation of tannins contents
The amount of tannins was estimated as the difference between total phenolic and non-complex residual phenol level after precipitation out with casein. Briefly, 0.5 g of casein was dissolved in  of distilled water, and 6.0 ml of the stock solution was added into the flask. The mixture was stirred for 3 h at room temperature. The non-complex residual phenol was separated by filtration. The filtrate was adjusted to 50.0 ml and used for estimation the phenolic contents using Folin-Ciocalteu method.
of distilled water, and 6.0 ml of the stock solution was added into the flask. The mixture was stirred for 3 h at room temperature. The non-complex residual phenol was separated by filtration. The filtrate was adjusted to 50.0 ml and used for estimation the phenolic contents using Folin-Ciocalteu method.
2.4. Estimation of flavonoid contents
The flavonoid content was estimated by an aluminum chloride colorimetric method [23]. Typically, a mixed solution of 0.5 ml of the stock solution and 1.7 ml of 1%  reagent was stirred and kept at room temperature for 10 min. The absorbance was measured at 415 nm using a UV-visible spectrophotometer. A standard quercetin solution
reagent was stirred and kept at room temperature for 10 min. The absorbance was measured at 415 nm using a UV-visible spectrophotometer. A standard quercetin solution  was used for plotting a standard curve. The total flavonoid contents of the extract were expressed as quercetin equivalent (mg quercetin/g crude extract).
was used for plotting a standard curve. The total flavonoid contents of the extract were expressed as quercetin equivalent (mg quercetin/g crude extract).
2.5. Preparation of  nanoparticles using guava leave extract
nanoparticles using guava leave extract
The reaction was carried out under nitrogen atmosphere. Typically, 0.9 g of the crude extract was dissolved in 20.0 ml of deionized water, and the solution was adjusted to pH 8 by adding 1.0 M of NaOH. Then 20.0 ml of aqueous solution of  was added into the extract. The mixture was stirred at room temperature for different reaction times. The obtained colloidal suspension was directly used as catalyst for methylene blue removal in aqueous solution. For characterization, the obtained nanoparticles were separated from the colloids by centrifuging and washing for several times with deionized water and ethanol. The obtained
was added into the extract. The mixture was stirred at room temperature for different reaction times. The obtained colloidal suspension was directly used as catalyst for methylene blue removal in aqueous solution. For characterization, the obtained nanoparticles were separated from the colloids by centrifuging and washing for several times with deionized water and ethanol. The obtained  nanoparticles were kept in a nitrogen atmosphere.
nanoparticles were kept in a nitrogen atmosphere.
2.6. Characterization
The particle sizes and morphology of the synthesized  nanoparticles were characterized using transmission electron microscopy (TEM). TEM images were obtained with a TECNAI 20 (Philips, USA) operated at 200 keV. Fourier transform infrared spectroscopy (FTIR) analysis was also performed on a universal attenuated total reflectance (ATR) accessory (PerkinElmer Frontier). The UV–vis spectroscopic absorbance was taken using UV–vis spectrophotometer model 8453 (Agilent Technologies, USA). Dynamic light scattering (DLS) measurement was carried out using Zeta Potential Analyzer (model S4700, Malvern Instrument, UK).
nanoparticles were characterized using transmission electron microscopy (TEM). TEM images were obtained with a TECNAI 20 (Philips, USA) operated at 200 keV. Fourier transform infrared spectroscopy (FTIR) analysis was also performed on a universal attenuated total reflectance (ATR) accessory (PerkinElmer Frontier). The UV–vis spectroscopic absorbance was taken using UV–vis spectrophotometer model 8453 (Agilent Technologies, USA). Dynamic light scattering (DLS) measurement was carried out using Zeta Potential Analyzer (model S4700, Malvern Instrument, UK).
2.7. Degradation of methylene blue dye
In a 10 ml glass vial, 5.0 ml of the fresh colloidal suspension of the synthesized  nanoparticles was added into 5.0 ml of the methylene blue solution (10, 20 and
nanoparticles was added into 5.0 ml of the methylene blue solution (10, 20 and  ). Then, the vials were rotated and agitated at different reaction times using a rolling machine. The degradation was studied in batch process at room temperature. The colloidal suspension was separated from the reaction mixture using centrifugation and the clear solution was monitored by UV–Vis spectroscopy at
). Then, the vials were rotated and agitated at different reaction times using a rolling machine. The degradation was studied in batch process at room temperature. The colloidal suspension was separated from the reaction mixture using centrifugation and the clear solution was monitored by UV–Vis spectroscopy at  of 664 nm.
of 664 nm.
3. Results and discussion
3.1. Guava leaf extract
Plant extract contains polyphenolic compounds which have been reported as an efficient reducing and protecting ability for converting metal ions to corresponding metal nanoparticles [24]. An efficient extract for the synthesis of metal nanoparticles should have sufficient reduction potential and can act as an excellent stabilizer to protect the freshly formed particles from aggregation. One of the most important factors to obtain extracts enriched in polyphenolic compounds is the type extraction solvent used. Polar solvents such as water and ethanol have been used for the extraction of polyphenolic compounds. The H-bonding of the polar solvent renders easier to interact with the phenol groups while the organic phenolic moieties interact well with non-polar solvents. As a result, the optimal value between polar and non-polar solvency is required to extract the polyphenolic content with maximal efficiency. Thus, the influence of solvents with different polarities on the extraction yield was thus investigated using water and ethanol mixture. The yields of crude extract (%) obtained from different extraction solvents are listed in table 1. The results showed that 70% hydroethanolic extract gives the highest yield crude extract of  while the pure water extract has only
while the pure water extract has only  . These data demonstrate that the yield of the extraction strongly depends on the polarities of the solvents.
. These data demonstrate that the yield of the extraction strongly depends on the polarities of the solvents.
Table 1. Yield of crude extract form guava leaf extract.
| Extraction solvent | Solvent polarity index (PI) | Yield crude eExtract (%) |
|---|---|---|
| Water | 9.0 | 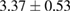 |
| 30% EtOH | 7.9 | 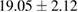 |
| 50% EtOH | 7.1 | 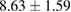 |
| 70% EtOH | 7.3 | 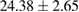 |
| Absolute EtOH | 5.2 | 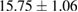 |
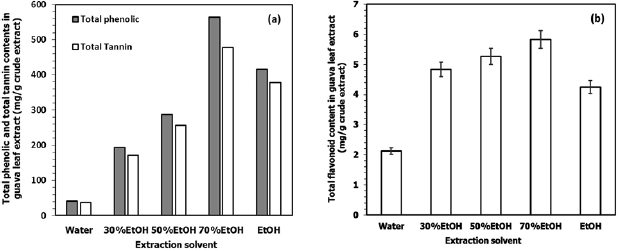
The total content of phenolic compounds, flavonoids and tannins from the crude extract was also determined. Figure 1(a) shows that guava leaf extract contains a high content of polyphenolic compounds. More than 90% of the total polyphenols are found to be the tannins. Absolute ethanol extract contains much higher content of tannins, which are 10 times higher than that of the water extract. However, the maximum content of tannins obtained is by the use of 70% hydroethanolic extract, which accounts for  of the crude extract. Guava leaf extract has much lower contents of total flavonoids compared with tannins. The maximum flavonoid content is also obtained from using 70% hydroethanolic extract, but only
of the crude extract. Guava leaf extract has much lower contents of total flavonoids compared with tannins. The maximum flavonoid content is also obtained from using 70% hydroethanolic extract, but only  crude extract is achieved (figure 1(b)). These results mean that hydroethanolic extract is more suitable for the extraction polyphenolic compound from guava leaves than pure water or absolute ethanol, and 70% hydroethanolic extract is the best extraction solvent. The results are consistent with previous reports of using mixed solvents [22, 25]. However, the optimum ratios of water and ethanol used here are different from these reports, which are likely dependent on the actual chemical nature of the compounds therein. Seo et al [22] reported that the best extraction solvent for using with guava leaves is 50% hydroethanolic extract. Qian et al [25] reported that the phenolic compound content obtained from 50% hydroethanolic extract is higher than water. Díaz-de-Cerio et al [26] presented that the highest amount of phenolic compounds resulted from using
crude extract is achieved (figure 1(b)). These results mean that hydroethanolic extract is more suitable for the extraction polyphenolic compound from guava leaves than pure water or absolute ethanol, and 70% hydroethanolic extract is the best extraction solvent. The results are consistent with previous reports of using mixed solvents [22, 25]. However, the optimum ratios of water and ethanol used here are different from these reports, which are likely dependent on the actual chemical nature of the compounds therein. Seo et al [22] reported that the best extraction solvent for using with guava leaves is 50% hydroethanolic extract. Qian et al [25] reported that the phenolic compound content obtained from 50% hydroethanolic extract is higher than water. Díaz-de-Cerio et al [26] presented that the highest amount of phenolic compounds resulted from using  80:20 (v/v) mixture for the extraction.
80:20 (v/v) mixture for the extraction.
Figure 1. (a) Total phenolic compounds and total tannins content, (b) total flavonoids content in guava leaf extract from different ratios of hydroethanolic solvent.
Download figure:
Standard image High-resolution imageFigure 2 shows UV-visible spectra of an aqueous solution of the crude extract at different pH values. The extract without adjusting pH (pH = 5) shows a broad absorption peak at the wavelength of 275 nm, which is assigned to the characteristic absorption of the phenolic groups. The sample with the pH adjusted to 3 (by adding 0.1 M HCl) shows a significant decrease in the absorption intensity. While an increase of pH from 5 to 10, there is a shift in the characteristic peak of a phenolic group to a longer wavelength at about 279 nm. The red shift of this peak is believed to be due to the delocalization of the π-electrons of phenoxides. It is thought that most of the ligands remain protonated at lower pH and the deprotonation increases at higher pH [27]. However, the absorption intensity decreases at pH 10. This change may be due to partially precipitation of the extract because of the use of a high ionic strength of the solution. The results suggest that by adjusting pH of guava leaf extract to 8 could provide a maximum extent of the deprotonated phenolic groups of tannins for complex formation, reduction, and stabilization of zero valent iron from iron ions.
Figure 2. Absorption spectra of guava crude extract in an aqueous solution at different pH values.
Download figure:
Standard image High-resolution image3.2. Complexation and formation of  nanoparticles
nanoparticles
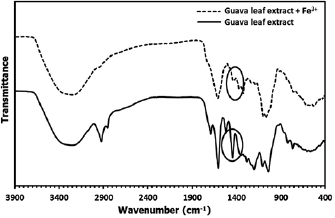
Guava leaf extract contains a high content of tannins with plenty of phenolic–OH of galloyl groups. These functional groups can form a strong complex with  ion. The comparison of FTIR spectra between pure guava leaf extract and its complexation with
ion. The comparison of FTIR spectra between pure guava leaf extract and its complexation with  ion are shown in figure 3.
ion are shown in figure 3.
Figure 3. FTIR spectrum of guava leaf extract and its  phenolic complex.
phenolic complex.
Download figure:
Standard image High-resolution imageThe infrared spectra of guava leaf extract show a strong and broad absorption band in the range  , corresponding to the hydroxyl groups (O–H) stretching vibrations. This indicates the presence of alcoholic and phenolic groups with a wide variety of hydrogen bonding [28]. A sharp peak at
, corresponding to the hydroxyl groups (O–H) stretching vibrations. This indicates the presence of alcoholic and phenolic groups with a wide variety of hydrogen bonding [28]. A sharp peak at  and
and  associated with –C–H stretching vibrations of
associated with –C–H stretching vibrations of  and –CH groups, indicating the presence aromatic ring (Ar–H) and glucose moieties
and –CH groups, indicating the presence aromatic ring (Ar–H) and glucose moieties  in tannins. The broad band in range
in tannins. The broad band in range  and
and  assigned to O–H in plane and out of plane blending, respectively [29]. The sharp peak at
assigned to O–H in plane and out of plane blending, respectively [29]. The sharp peak at  is attributed to the C=C stretching vibration in the phenolic groups. A small peak at
is attributed to the C=C stretching vibration in the phenolic groups. A small peak at  assigned to carbonyl (–C=O) group indicates that an ester bond is formed between two galloyl groups. The peak at
assigned to carbonyl (–C=O) group indicates that an ester bond is formed between two galloyl groups. The peak at  is assigned to the C–O stretching vibration of phenolic groups. In comparison with the spectrum of the extract, the spectrum of the mixture between
is assigned to the C–O stretching vibration of phenolic groups. In comparison with the spectrum of the extract, the spectrum of the mixture between  and the extract is similar, but the intensity of the peak in range 1200 to
and the extract is similar, but the intensity of the peak in range 1200 to  of O–H in plane bending becomes much decreased. The significant changes of spectrum confirm that the deprotonation and interaction between
of O–H in plane bending becomes much decreased. The significant changes of spectrum confirm that the deprotonation and interaction between  and o-dihydroxyphenyl groups on phenolic compounds and complexation are therefore taken place [30].
and o-dihydroxyphenyl groups on phenolic compounds and complexation are therefore taken place [30].
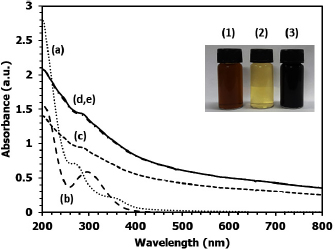
Formation of  nanoparticles can be monitored by UV–vis absorption spectroscopy. Figure 4 shows the absorption spectra of guava leaf extract (spectrum a) and an aqueous
nanoparticles can be monitored by UV–vis absorption spectroscopy. Figure 4 shows the absorption spectra of guava leaf extract (spectrum a) and an aqueous  solution (spectrum b) compared to the absorption spectra of
solution (spectrum b) compared to the absorption spectra of  nanoparticles prepared at different reaction times (spectra c, d and e). Guava leaf extract shows characteristic peaks of the phenolic group at the wavelength of 275 nm while an aqueous solution of
nanoparticles prepared at different reaction times (spectra c, d and e). Guava leaf extract shows characteristic peaks of the phenolic group at the wavelength of 275 nm while an aqueous solution of  precursor exhibits a bright yellowish colour with a broad peak at 301 nm of
precursor exhibits a bright yellowish colour with a broad peak at 301 nm of  [30]. After mixing, the colour of mixture immediately changes to dark blue/green due to the complexation of
[30]. After mixing, the colour of mixture immediately changes to dark blue/green due to the complexation of  with the phenolic groups of the extract [27]. The effect of reaction time on the nucleation and growth of
with the phenolic groups of the extract [27]. The effect of reaction time on the nucleation and growth of  nanoparticles was investigated. At reaction time 5 min, spectrum c displayed an exponential decaying profile as the wavelength increased. The exponential shape is characteristic of a band like electronic structure, which suggests the formation of
nanoparticles was investigated. At reaction time 5 min, spectrum c displayed an exponential decaying profile as the wavelength increased. The exponential shape is characteristic of a band like electronic structure, which suggests the formation of  clusters. In general, colloidal dispersions of metals typically exhibit absorption bands due to surface plasmon resonance or broad regions of absorption in the ultraviolet–visible range [31]. Thus, the result demonstrates that guava leaf extract has high reduction potential to reduce
clusters. In general, colloidal dispersions of metals typically exhibit absorption bands due to surface plasmon resonance or broad regions of absorption in the ultraviolet–visible range [31]. Thus, the result demonstrates that guava leaf extract has high reduction potential to reduce  to
to  in a short time at room temperature. With an increase in reaction time to 12 h (spectrum d), there is an increase in the absorption intensity due to the growth process of nanoparticles. There is no change in the intensity of the absorbance spectrum when the reaction time reaches 24 h (spectrum e). This result suggests that good stability of the obtained
in a short time at room temperature. With an increase in reaction time to 12 h (spectrum d), there is an increase in the absorption intensity due to the growth process of nanoparticles. There is no change in the intensity of the absorbance spectrum when the reaction time reaches 24 h (spectrum e). This result suggests that good stability of the obtained  nanoparticles since the absorption intensity would decrease in the case of agglomeration or aggregation.
nanoparticles since the absorption intensity would decrease in the case of agglomeration or aggregation.
Figure 4. UV-visible absorption spectra of (a) guava leaf extract, (b) aqueous solution of 0.01 M  ; (c)
; (c)  nanoparticles prepared at room temperature for (c) 5 min, (d) 12 h and (e) 24 h at pH 8. Inset: the photograph of (1) guava leaf extract, (2)
nanoparticles prepared at room temperature for (c) 5 min, (d) 12 h and (e) 24 h at pH 8. Inset: the photograph of (1) guava leaf extract, (2)  solution and (3) colloidal
solution and (3) colloidal  nanoparticles.
nanoparticles.
Download figure:
Standard image High-resolution imageReaction time is an important factor affecting the particle size and morphology of nanoparticles. Figures 5(a) and (c) show TEM images and absorption spectra of the synthesized tannins-stabilized  nanoparticles prepared with the concentration of
nanoparticles prepared with the concentration of  0.01 M for 24 and 48 h. At 24 h, the obtained Fe nanoparticles are small with a narrow size distribution. With increasing the reaction time from 24 to 48 h, the average particle size increases from
0.01 M for 24 and 48 h. At 24 h, the obtained Fe nanoparticles are small with a narrow size distribution. With increasing the reaction time from 24 to 48 h, the average particle size increases from  to
to  , respectively. In general, the particle size of nanoparticles can be adjusted by controlling their nucleation and growth rate by using different concentrations of metal ion precursor, which is one of the important experimental factors [11]. With increasing the concentration of
, respectively. In general, the particle size of nanoparticles can be adjusted by controlling their nucleation and growth rate by using different concentrations of metal ion precursor, which is one of the important experimental factors [11]. With increasing the concentration of  from 0.01 M to 0.05 M, the average particle size of
from 0.01 M to 0.05 M, the average particle size of  nanoparticles is found to be slightly increased to
nanoparticles is found to be slightly increased to  (figure 5(e)). The formation of
(figure 5(e)). The formation of  nanoparticles can be monitored from its absorption band. From figures 5(b), (c) and (f), it can be seen that the absorption intensity depends on its particle size. With smaller size of Fe nanoparticles, higher absorption intensity in UV-visible region is obtained implying the formation of higher surface area of Fe clusters. In the growth process, the growth of particle occurs due to the collision of nuclei/particles with chelated iron atoms [32]. Thus, the use of low concentration of
nanoparticles can be monitored from its absorption band. From figures 5(b), (c) and (f), it can be seen that the absorption intensity depends on its particle size. With smaller size of Fe nanoparticles, higher absorption intensity in UV-visible region is obtained implying the formation of higher surface area of Fe clusters. In the growth process, the growth of particle occurs due to the collision of nuclei/particles with chelated iron atoms [32]. Thus, the use of low concentration of  precursor results in less collision hence, accounting for the formation of small Fe nanoparticles. At higher concentration of
precursor results in less collision hence, accounting for the formation of small Fe nanoparticles. At higher concentration of  , the incorporation efficiency of atoms onto nuclei/particles would be higher per collision resulting in larger particle size. A slightly change of the particle size when the concentration of
, the incorporation efficiency of atoms onto nuclei/particles would be higher per collision resulting in larger particle size. A slightly change of the particle size when the concentration of  precursor is increased results from strong protection of the extract. There is no sign of chain-like aggregation due to the van der Waals interactions and magnetic properties that normally occur on Fe nanoparticles. Thus, the results clearly demonstrate the high stabilization ability of guava leaf extract.
precursor is increased results from strong protection of the extract. There is no sign of chain-like aggregation due to the van der Waals interactions and magnetic properties that normally occur on Fe nanoparticles. Thus, the results clearly demonstrate the high stabilization ability of guava leaf extract.
Figure 5. TEM images and absorption spectra of tannins stabilized Fe nanoparticles prepared at room temperature for different time and concentration of  precursor, respectively; (a) and (b) 24 h and 0.01 M, (c) and (d) 48 h and 0.05 M, (e) and (f) 24 h and 0.05 M.
precursor, respectively; (a) and (b) 24 h and 0.01 M, (c) and (d) 48 h and 0.05 M, (e) and (f) 24 h and 0.05 M.
Download figure:
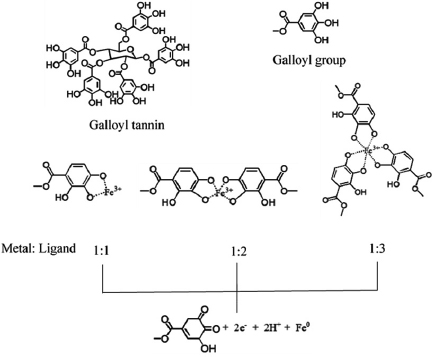
Standard image High-resolution imageTannins containing phenolic-OH of galloyl groups can form highly stable complexes with  [33]. The stoichiometry and stability of the complex depend on pH and its pKa value. Tannins give a pKa value between seven and eight and are well-known to hydrolyze partially under mild acidic/basic conditions into glucose and gallic acid units. This experiment, pH of the extract was adjusted to 8 before mixing with an aqueous solution of
[33]. The stoichiometry and stability of the complex depend on pH and its pKa value. Tannins give a pKa value between seven and eight and are well-known to hydrolyze partially under mild acidic/basic conditions into glucose and gallic acid units. This experiment, pH of the extract was adjusted to 8 before mixing with an aqueous solution of  (pH = 2), then the pH of the mixture (about 5) was adjusted to pH 8. At low pH, most of the ligands may remain protonated, and only a few binding sites might be available for the
(pH = 2), then the pH of the mixture (about 5) was adjusted to pH 8. At low pH, most of the ligands may remain protonated, and only a few binding sites might be available for the  ions. Whereas, an increase of pH to 8, the deprotonation increased and provided more binding sites for
ions. Whereas, an increase of pH to 8, the deprotonation increased and provided more binding sites for  . Phenolic-OH of a galloyl group has a different capability to bind metal ions due to several binding sites. The concentration of metal ion thus dictates the final stoichiometry of the complex. With high
. Phenolic-OH of a galloyl group has a different capability to bind metal ions due to several binding sites. The concentration of metal ion thus dictates the final stoichiometry of the complex. With high  concentration, one
concentration, one  ion may chelate to one galloyl group through two sites of o-dihydroxyphenyl groups while it may chelate to two or three galloyl groups (4 and 6 binding sites) at low concentration [7]. The interaction between
ion may chelate to one galloyl group through two sites of o-dihydroxyphenyl groups while it may chelate to two or three galloyl groups (4 and 6 binding sites) at low concentration [7]. The interaction between  and o-dihydroxy- phenyl groups can take part in redox reactions to form quinones and donate electrons, due to the complexation of adjacent hydroxyl groups. Complexation and reduction mechanism of galloyl group is shown in figure 6. Cakar et al [34] proposed the possible ratio of metal to ligand of Fe-tannic acid complexes. At pH lower than 2, the favor ratio was 1:1 complexation. While at 3 < pH < 6 and higher than 7, the favored metal to ligand ratios were 1:2 and 1:3, respectively [34]. From the experiment, using a low ratio of the concentration of
and o-dihydroxy- phenyl groups can take part in redox reactions to form quinones and donate electrons, due to the complexation of adjacent hydroxyl groups. Complexation and reduction mechanism of galloyl group is shown in figure 6. Cakar et al [34] proposed the possible ratio of metal to ligand of Fe-tannic acid complexes. At pH lower than 2, the favor ratio was 1:1 complexation. While at 3 < pH < 6 and higher than 7, the favored metal to ligand ratios were 1:2 and 1:3, respectively [34]. From the experiment, using a low ratio of the concentration of  precursor to the extract (about 1:10),
precursor to the extract (about 1:10),  should from a strong complex to tannin with 1:3 ratio resulting in a strong reduction of
should from a strong complex to tannin with 1:3 ratio resulting in a strong reduction of  to
to  .
.
Figure 6. Complexation and reduction mechanism of galloyl group based on the reduction of  to
to  .
.
Download figure:
Standard image High-resolution image3.3. Stability of  nanoparticles in colloidal suspension
nanoparticles in colloidal suspension
The stability of  nanoparticles was evaluated by monitoring the sedimentation time of the nanoparticle suspension. All of the obtained tannins-stabilized
nanoparticles was evaluated by monitoring the sedimentation time of the nanoparticle suspension. All of the obtained tannins-stabilized  nanoparticles were remained suspended over 7 days with no noticeable of sedimentation or flocculation. Zeta
nanoparticles were remained suspended over 7 days with no noticeable of sedimentation or flocculation. Zeta  potential evaluates the stability of Fe nanoparticles in colloidal form. The zeta potential value for the prepared samples is in the range of −40 to −45 mV at neutral pH. This negative zeta potential arises due to the capping of the particles by hydroxyl groups of the polyphenolic compounds. This zeta potential value when maintained to be more negative than −30 mV can give long term stability and good colloidal stabilization and dispersity of tannins-stabilized
potential evaluates the stability of Fe nanoparticles in colloidal form. The zeta potential value for the prepared samples is in the range of −40 to −45 mV at neutral pH. This negative zeta potential arises due to the capping of the particles by hydroxyl groups of the polyphenolic compounds. This zeta potential value when maintained to be more negative than −30 mV can give long term stability and good colloidal stabilization and dispersity of tannins-stabilized  nanoparticles.
nanoparticles.
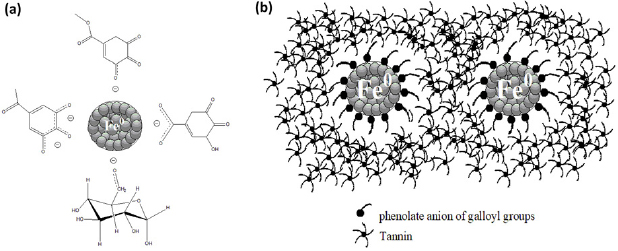
The role of guava leaf extract in stabilization  nanoparticles is considered as steric stabilization, from the interaction between the possible functional groups of tannins and Fe atoms on the surfaces. Tannins, the most phenolic compounds in guava leaf extract, can be hydrolyzed by weak acids or weak based to produce corresponding glucose and gallic acid moieties [35]. At pH 8, glucose and gallic acid may play an important role in stabilization. The nanoparticle surfaces could be stabilized by the electrostatic interaction of COO− of deprotonated gallic acid and the OH groups of the glucose moiety. The phenolic OH of galloyl groups act as hard ligand so favorable to form a strong complex with
nanoparticles is considered as steric stabilization, from the interaction between the possible functional groups of tannins and Fe atoms on the surfaces. Tannins, the most phenolic compounds in guava leaf extract, can be hydrolyzed by weak acids or weak based to produce corresponding glucose and gallic acid moieties [35]. At pH 8, glucose and gallic acid may play an important role in stabilization. The nanoparticle surfaces could be stabilized by the electrostatic interaction of COO− of deprotonated gallic acid and the OH groups of the glucose moiety. The phenolic OH of galloyl groups act as hard ligand so favorable to form a strong complex with  (hard metal ion). Then, each Fe nanoparticle may be stabilized by carbonyl group of quinone generated from reduction of phenolic-OH of galloyl groups. When soft ligand of C=O on quinone come to contact with the surface of hard metals (Fe particles), the poor stabilization is occurred [36]. Thus, the possible active groups that are responsible for the stabilization Fe nanoparticles in an alkaline solution of guava leaf extract should be phenolate anion of galloyl groups, OH group of glucose and carboxylate group (COO−) of gallic acid (figure 7(a)). Another possible role of the extract to stabilize
(hard metal ion). Then, each Fe nanoparticle may be stabilized by carbonyl group of quinone generated from reduction of phenolic-OH of galloyl groups. When soft ligand of C=O on quinone come to contact with the surface of hard metals (Fe particles), the poor stabilization is occurred [36]. Thus, the possible active groups that are responsible for the stabilization Fe nanoparticles in an alkaline solution of guava leaf extract should be phenolate anion of galloyl groups, OH group of glucose and carboxylate group (COO−) of gallic acid (figure 7(a)). Another possible role of the extract to stabilize  nanoparticles is 'depletion stabilization', which could occur due to a high concentration of the extract (figure 7(b)). Thus, aggregation could be inhibited by the presence of free protecting agents due to the creation of high-energy depletion zones between closely interacting particle surfaces [37].
nanoparticles is 'depletion stabilization', which could occur due to a high concentration of the extract (figure 7(b)). Thus, aggregation could be inhibited by the presence of free protecting agents due to the creation of high-energy depletion zones between closely interacting particle surfaces [37].
Figure 7. Schematic representation the stabilization of  nanoparticles by guava leaf extract (a) steric stabilization and (b) depletion stabilization.
nanoparticles by guava leaf extract (a) steric stabilization and (b) depletion stabilization.
Download figure:
Standard image High-resolution image3.4. Application of tannins-stabilized  nanoparticles towards the degradation of methylene blue
nanoparticles towards the degradation of methylene blue
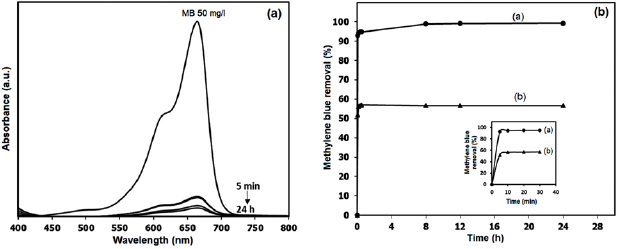
Methylene blue degradation in an aqueous solution was performed as a model for study catalytic reduction of the synthesized tannin-stabilized  nanoparticles in colloidal form. The percentage of methylene blue removal at different reaction times is shown in figure 8(a) as compared to the decrease in adsorption intensity of methylene blue spectrum in figure 8(b). The results demonstrate that the synthesized tannins-stabilized
nanoparticles in colloidal form. The percentage of methylene blue removal at different reaction times is shown in figure 8(a) as compared to the decrease in adsorption intensity of methylene blue spectrum in figure 8(b). The results demonstrate that the synthesized tannins-stabilized  nanoparticles have the ability to remove methylene blue from the solution. From the reaction condition, nearly 100% methylene blue molecules can be removed.
nanoparticles have the ability to remove methylene blue from the solution. From the reaction condition, nearly 100% methylene blue molecules can be removed.
Figure 8. (a) Changes in the absorption spectrum of methylene blue with reaction time. (b) The percentage of methylene removals at different reaction times. The initial time is shown inset. The concentration of methylene blue is  , the amount of colloidal tannin-stabilized
, the amount of colloidal tannin-stabilized  nanoparticles is
nanoparticles is  with about 11 wt% of Fe nanoparticles (from theoretical calculation).
with about 11 wt% of Fe nanoparticles (from theoretical calculation).
Download figure:
Standard image High-resolution imageThe absorption spectra in figure 8(a) identified that most of methylene blue molecules are removed using the colloidal suspension of tannin-stabilized  nanoparticles during 24 h with a sharp decrease in 5 min. In figure 8(b) the curve (a) represents the methylene blue removal by the colloidal suspension of tannins-stabilized
nanoparticles during 24 h with a sharp decrease in 5 min. In figure 8(b) the curve (a) represents the methylene blue removal by the colloidal suspension of tannins-stabilized  nanoparticles, and the curve (b) represents the methylene blue removal by the
nanoparticles, and the curve (b) represents the methylene blue removal by the  nanoparticles green synthesized in the pure guava leaf extract. In the case of using colloidal suspension of tannins-stabilized
nanoparticles green synthesized in the pure guava leaf extract. In the case of using colloidal suspension of tannins-stabilized  nanoparticles the methylene blue removal rapidly achieved the value 99%, while in the case of using
nanoparticles the methylene blue removal rapidly achieved the value 99%, while in the case of using  nanoparticles green synthesized in the pure guava leaf extract the methylene blue removal can achieve only value 50%. The removal of methylene blue from pure guava leaf extract may occur due to the interaction between hydroxyl group of phenolic compounds and cationic species of methylene blue. While the higher removal ability in case of using tannin-stabilized
nanoparticles green synthesized in the pure guava leaf extract the methylene blue removal can achieve only value 50%. The removal of methylene blue from pure guava leaf extract may occur due to the interaction between hydroxyl group of phenolic compounds and cationic species of methylene blue. While the higher removal ability in case of using tannin-stabilized  nanoparticles may not only from adsorption but also from decolorization and degradation process. At initial time, decrease of methylene blue color may result from decolorization.
nanoparticles may not only from adsorption but also from decolorization and degradation process. At initial time, decrease of methylene blue color may result from decolorization.  nanoparticles can transfer an electron to methylene blue and turn to leuco methylene blue which in the colorless form. However adsorption and decolorization process take place in short time. The color will slowly return to blue when contact to air. In this experiment, there is no sign of returning to the original color. This may possible that the polyphenolic groups which stabilize
nanoparticles can transfer an electron to methylene blue and turn to leuco methylene blue which in the colorless form. However adsorption and decolorization process take place in short time. The color will slowly return to blue when contact to air. In this experiment, there is no sign of returning to the original color. This may possible that the polyphenolic groups which stabilize  nanoparticles and can act as antioxidant by provide electron to Fe atom. In addition, disappearing color permanent may result from high methylene blue degradation activity of the synthesized
nanoparticles and can act as antioxidant by provide electron to Fe atom. In addition, disappearing color permanent may result from high methylene blue degradation activity of the synthesized  nanoparticles at initial time. From the graph of methylene blue removal (figure 8(b)), there is a continuous increase in the percentage of methylene blue removal from 94 to 99 during 5 to 24 h. The results indicate that methylene blue can be removed due to its chemical reduction by tannins-stabilized
nanoparticles at initial time. From the graph of methylene blue removal (figure 8(b)), there is a continuous increase in the percentage of methylene blue removal from 94 to 99 during 5 to 24 h. The results indicate that methylene blue can be removed due to its chemical reduction by tannins-stabilized  nanoparticles and adsorption. High reactivity of the synthesized
nanoparticles and adsorption. High reactivity of the synthesized  nanoparticles associated its high surface areas and dispersion stability. Reactivity of iron-based nanoparticles synthesized by tea extract to the degradation of methylene blue has been reported and the degraded products such as benzothiazole compounds have been previously identified [38]. With high activity, high particle dispersion, and environmentally friendly nanomaterials, the colloidal
nanoparticles associated its high surface areas and dispersion stability. Reactivity of iron-based nanoparticles synthesized by tea extract to the degradation of methylene blue has been reported and the degraded products such as benzothiazole compounds have been previously identified [38]. With high activity, high particle dispersion, and environmentally friendly nanomaterials, the colloidal  nanoparticles synthesized from guava leaf extract can apply for both in situ and ex situ remediation of other compounds especially highly stable chlorinated compounds.
nanoparticles synthesized from guava leaf extract can apply for both in situ and ex situ remediation of other compounds especially highly stable chlorinated compounds.
4. Conclusion
In summary, we have demonstrated that the guava leaf extract has high reduction potential and strong protecting ability to the preparation of  nanoparticles at room temperature in an aqueous solution. The maximum polyphenolic compounds can be obtained by using 70% hydroethanolic extract. Tannins constitute the most components of the polyphenolic compounds can form a strong complex with
nanoparticles at room temperature in an aqueous solution. The maximum polyphenolic compounds can be obtained by using 70% hydroethanolic extract. Tannins constitute the most components of the polyphenolic compounds can form a strong complex with  in basic solution, which further enhance their strong reduction potentials. Also, in basic solution, the phenoxide anion, hydroxyl group of glucose and carboxylate groups can strongly interact with the surface of Fe nanoparticles to offer the protection from the freshly made particles from aggregation. Colloidal tannins-stabilized
in basic solution, which further enhance their strong reduction potentials. Also, in basic solution, the phenoxide anion, hydroxyl group of glucose and carboxylate groups can strongly interact with the surface of Fe nanoparticles to offer the protection from the freshly made particles from aggregation. Colloidal tannins-stabilized  nanoparticles prepared from guava leaf extract is thus demonstrated to offer a high activity towards the degradation of methylene blue.
nanoparticles prepared from guava leaf extract is thus demonstrated to offer a high activity towards the degradation of methylene blue.
Acknowledgments
The authors gratefully acknowledge the Thailand Research Fund (TRF), Office of the Higher Education Commission (Thailand) and Faculty of Science Burapha University for financial support.