Abstract
Zinc oxide (ZnO) and titanium dioxide (TiO2) nanoparticles (NPs) are prepared from their salts in form of aqueous suspensions to provide multifunctional cellulosic substrate. ZnO-NPs and TiO2-NPs are obtained in the range of 3–5 nm and 8–15 nm, respectively. Prepared suspensions are loaded onto knitted cotton fabrics and fixed using reactive softener and/or citric acid. Antimicrobial activity, ultraviolet protection (UPF) and self-cleaning in respect to metal oxides add-on to the fabrics are investigated. Fabrics treated with ZnO-NPs for 10 g l−1 (white fabrics) and 5 g l−1 (dyed fabrics) were cidal for both gram negative and positive bacteria. Using 5 g l−1 TiO2-NPs in the presence of reactive softener has a bacteriostatic effect and showed a decent bacteria reduction. TiO2-NPs showed the better UPF values than those of ZnO treated fabrics when it compared to the same fabric structure. Discoloration values of TiO2-NPs treated fabrics showed superior color fading over the ZnO-NPs treated samples.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanoscience and nanotechnology, new directions of modern research, have emerged during the past decades. This trend involves the ability to fabricate, characterize and manipulate artificial materials whose features are controlled at the nano-scale. Studies of the properties of these nano-scale materials convincingly demonstrate their unique biocidal, optical, chemical and many other potentials [1].
The research interest of using inorganic nanoparticles in producing multifunctional substrates has increased due to their ability to develop substrates with different functional characteristics, stability against processing conditions, less energy consumption with eco-friendly consideration [2].
Among these inorganic nanoparticles, metal oxides such as zinc oxide and titanium dioxide have shown a great importance in various applications due to their capability of absorbing light and transporting charges with the formation of positive electron-hole pairs that are able to oxidize organic substrates [3]. Therefore, loading of such metal oxides nanoparticles into natural platforms such as cellulose substrates [4] have been anticipated for various applications in food packaging and textiles [5].
TiO2-NPs and ZnO NPs show huge advantageous properties over many others in terms of their ease of preparation from cheap salts through conventional procedures, their independency of loaded substrate material, their ease of processing, potential of large-scale manufacturing and their multifunctionality introduced to the loaded substrates [6]. In particular, the reduction of such metal salts could be engineered to produce nano-structured particles which lead to a higher surface area. The former facility will provide new generation of cheap finishing agents that require less energy in preparation and applications with great potentials.
TiO2-NPs have received significant interest in recent years due to its fascinating properties, such as electronic, UV absorption, its brightness and photo-catalyst. Nano-structured TiO2 has been considered as a promising catalyst for decomposition of organic contaminants and microorganisms which leads to an amazing property to the treated substrate such as self-cleaning material, air and water purifications and environmental sterilization [7, 8]. Titanium dioxide, known by titania, is been frequently used in the production of paints, plastic, welding rod-coating materials and cosmetics [7, 9, 10]. Therefore, methods used for its preparation such as auto-combustion, thermo-hydrolysis, thermal plasma process, and sol–gel methods [10] do not match the cellulosic substrate processing steps. Accordingly, the nano-crystalline titanium dioxide is generally prepared by hydrolysis titanium precursors in very controlled water phase as described by Tung [9].
ZnO-NPs are a very interesting multifunctional material because of its promising application in whiteness and UV-blocking property as well as anti-microbial functionality. ZnO-NPs which have high surface area and intense absorption in UV region have been known as UV-blocker that more stable than any other organic agents [11, 12]. Different methods have been reported for the preparation of ZnO-NPs such as; the sol-gel technique, the solutions and suspensions evaporation, evaporative decomposition of solutions, conventional ceramic fabrication, and the wet chemical processes [12].
The biggest challenges face fabrics, treated with such metal oxides nanoparticles, are the stabilization of the treatment emulsions for prolonged time and the durability of the treatment on the fabric for long-term use [13]. Therefore, different stabilizing agents have been employed to obtain stable metal oxide emulsions for long periods during the preparation process. For instance, ethylene glycol [14] and hyperbranched poly (amide-amine) [15] were used as stabilizing agents.
However, acrylic binders have been used (1%) as fixing agent after fabric treatment in order to enhance the fixation of such nanoparticles onto fabrics.
In the current study, TiO2-NPs and ZnO NPs are prepared, in the presence of soluble starch for stabilization, from their salts in the form of aqueous suspensions. The influence of the type and concentration of metal-oxide nanoparticles (MO-NPs), fabric construction and finishing ingredients were studied in order to obtain multi-functional knitted cotton fabrics.
2. Experimental
2.1. Materials and methods
Zinc acetate, poly ethylene glycol (DP 600) (PEG-600) and titanium tetraisopropoxide (TTIP) were purchased from Alfa Aesar. Reactive silicone softener (GB-silicon; BASF) was provided as amino functional silicones. Soluble starch (SS) was purchased from Acros Organics. All were of general laboratory grade. Also, knitted fabrics were used as white and dyed by Reactive Orange 127 (hetero-bifunctional reactive dye). Specifications of the knitted fabrics used are as follows: Milton  Peque
Peque  ; Jersey 20/1
; Jersey 20/1  .
.
2.2. Aqueous emulsion of zinc oxide
2.2.1. Zinc oxide nanoparticles.
ZnO NPs were prepared as described in previous work [2] through a chemical co-precipitation method using zinc acetate as a precursor and sodium hydroxide (0.25 M) in the presence of soluble starch (SS). In typical procedure, the dissolution of 14.3 g zinc acetate,  in 500 ml distilled water was taken place in addition to 5 g of SS. The solutions were exposed to microwave for 1 min. The addition of 500 ml of 0.25 M of sodium hydroxide solution was taken place dropwise and the mixture was left for stirring for couple of hours [11]. Samples were filtered and dialyzed against distilled water over 2 d to adjust pH to 7. Samples were dried at 90 °C for 4 h before calcinations process which was carried out at 400 °C for 3 h. ZnO-NPs reported for this procedure are in the range of 3–5 nm.
in 500 ml distilled water was taken place in addition to 5 g of SS. The solutions were exposed to microwave for 1 min. The addition of 500 ml of 0.25 M of sodium hydroxide solution was taken place dropwise and the mixture was left for stirring for couple of hours [11]. Samples were filtered and dialyzed against distilled water over 2 d to adjust pH to 7. Samples were dried at 90 °C for 4 h before calcinations process which was carried out at 400 °C for 3 h. ZnO-NPs reported for this procedure are in the range of 3–5 nm.
2.2.2. Zinc oxide emulsion.
Zinc oxide emulsion for fabric treatments was prepared in distilled water to obtain 2.5, 5 and 10 g l−1 in the presence of dispersing agent, 2 g l−1, PEG-600. Knitted cotton fabrics were treated with/without fixing agents such as citric acid/sodium hypophosphite or reactive softener (silicone based) (1% w.o.f) with 1% citric acid (acid catalyst). All ingredients were homogenized and sonicated for 2 h before padding the cotton fabrics.
2.3. Titanium dioxide finishing emulsion
2.3.1. Titanium dioxide nanoparticles.
In typical procedure, a 10 ml solution of 1 M TTIP in anhydrous ethanol was added slowly to a well stirred mixture of 1.5 ml water, 5 ml ethanol and 20 ml of anhydrous ethanol. The mixture was stirred overnight at room temperature. Excess ethanol was evaporated and titanium dioxide powder was dried at 90 °C for 4 h and calcinated at 700 °C for 2 h [10].
2.3.2. Titanium dioxide emulsion.
Titanium oxide emulsion for fabric treatments was prepared in distilled water to obtain 2.5, 5 and 10 g l−1 in the presence of 2 g l−1, dispersing agent, PEG-600. Knitted cotton fabrics were treated with/without fixing agents such as citric acid/sodium hypophosphite or reactive softener (silicone based) (1% w.o.f.)/citric acid (acid catalyst). All ingredients was homogenized and sonicated for 2 h before padding the knitted cotton fabrics.
2.4. Thermofixiation of the treated fabrics
Fixing agents were used to enhance the durability of the nanoparticles onto the fabric such as citric acid and reactive softeners. Fabrics treated with finishing agents emulsion, containing such fixing agents, were dried at 120 °C for 5 min and thermo-fixed at 160 °C for 3 min.
2.5. Characterization
2.5.1. Infrared spectroscopy (IR).
The FT-IR tester of Nicolet Magna-IR 560 spectrometer was used to analyze the spectrum of the untreated and treated samples. The tester collected transmittance of the infrared in the film between 400 and  are examined.
are examined.
2.5.2. Antimicrobial assay.
Antimicrobial potentials of the treated cotton fabrics were tested against bacterial strains E. coli ATCC 8379 and Staphylococcus aureus ATCC 25923 generously provided by Department of Microbiology, Faculty of Agriculture, Cairo University. The bacteria were preserved on brain heart infusion agar slants and stored at 4 °C and were re-cultured constantly each 3 months. For using in the experiment, brain heart infusion broth tubes were inoculated with a loop full of different bacterial culture on slants and incubated for 24 h at 37 °C and subcultured once more in broth for another 24 h at 37 °C [16].
Sterilized 0.1 g of each sample was inserted in a separated well in 24 wells microplate, and 2 ml of nutrient broth were added to each well, then inoculated with  of 24 h incubated bacterial culture diluted to have final concentration of
of 24 h incubated bacterial culture diluted to have final concentration of  . Two controls were performed, one with added untreated samples and the other with tested bacteria only.
. Two controls were performed, one with added untreated samples and the other with tested bacteria only.
After incubation for 24 h at 37 °C, bacterial count of each sample was determined using drop plate method (Naghili et al 2013) [17].
Samples with bacteristatic effect were defined as samples which showed no increase or decrease of the initial inoculation counting while bactericidal effect defined as samples showed no bacterial viability after 24 h of incubation at 37 °C [18].
2.5.3. Atomic absorption.
A Varian Model SpectrAA 220 multi-element flame atomic absorption spectrometer, equipped with a conventional pneumatic nebulizer and nebulization chamber was used to determine the metal content in the treated fabric samples. A multi-element hollow cathode lamp for determination of zinc and titanium was used. The most sensitive wavelengths for zinc at 213.9 nm and titanium at 364.3 nm were used with bandwidths of 1.0 and 0.5 nm respectively. The flame composition was acetylene (flow rate:  ) and air (flow rate:
) and air (flow rate:  ).
).
2.5.4. UPF analysis.
The UV-protection factor (UPF) of the treated and untreated samples was determined according to the Australian/New Zealand standard (AS/NZS 4366-1996).
2.5.5. Self-cleaning action.
The stain removal efficiency or self-cleaning capability of the treated samples was demonstrated as follow. The treated and untreated samples with ZnO and TiO2 were immersed in 0.02% (weight of fabric) of Methylene blue solution for 30 min and air-dried. Stained samples were exposed to sunlight for 24 h. Color measurements for the un/treated samples were carried out using a Perkin-Elmer Lambada 3B UV/Vis spectrophotometer. Color strength values (K/S) were determined using Kubelka-Munk equation.
3. Results and discussion
Preparation of zinc oxide nanoparticles which hydrolyzed via sodium hydroxide in the presence of different capping agents namely; lactose, soluble starch, PVP, and urea were studied in our laboratory. Also, ZnO-NPs prepared samples were characterized via TGA, X-RD, TEM and IR and data interpreted in our previous work [2].
3.1. Thermogravimetric analysis (TGA)and x-ray diffraction (XRD)
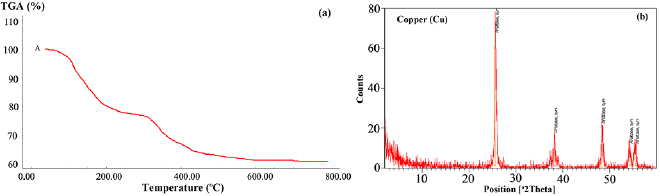
TGA analysis was carried out to dried samples of hydrolyzed titanium tetra isopropoxide (TTIP), diluted in anhydrous ethanol, via controlled addition of water/acetic acid to investigate the decomposition processes of titanium precursor (TTIP) at elevated temperature span from 25 °C to 600 °C. Figure 1(a) shows the TGA data which reveals three main stages of TiO2 decomposition observed in the heat profile. In the first stage, temperature increases from 25 °C to 180 °C in which the remaining ethanol and water have removed and about 20% of weight loss was taken place. In the second stage of temperature profile (180 °C–380 °C), it refers to the decomposition of 90% of the organic compounds. It is clear that the anatase phase was obtained in temperature range 425 °C–500 °C [19] as shown in XRD results in figure 1(b).
Figure 1. (a) TGA charts of hydrolyzed TiO2 via water/acetic acid; (b) X-RD charts for TiO2 hydrolyzed via distilled water.
Download figure:
Standard image High-resolution imageThe x-ray diffraction was used to define the phase composition of TiO2 and the crystallite size. Figure 1(b) shows the X-RD of calcinated TiO2 powder at 600 °C. The peaks were identified according 2Theta which confirmed that an anatase structure of TiO2 was formed at 2Theta = 26°. The peaks obtained for reflection of  particles were very close to the reported data [19]. The
particles were very close to the reported data [19]. The  spacing values 3.4, 2.3, 1.8, 1.68 and 1.65 and their relative intensities at °2Theta positions 25.6, 38.18, 48.42, 54.31 and 55.55 respectively which coincide to the published data of Anatase [20].
spacing values 3.4, 2.3, 1.8, 1.68 and 1.65 and their relative intensities at °2Theta positions 25.6, 38.18, 48.42, 54.31 and 55.55 respectively which coincide to the published data of Anatase [20].
3.2. Transmission electron microscopy (TEM)
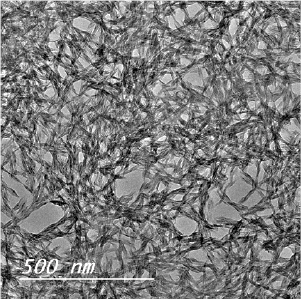
Figure 2 shows the surface morphology of TiO2-NPs hydrolyzed by water/acetic acid. TiO2-NPs hydrolyzed by water/acetic acid system shows a hexagonal complex of needle-shaped nano-particles in nano-sized length with ranges from 8 to 15 nm.
Figure 2. TEM images for dried TiO2 particles hydrolyzed by hydrogen peroxide (B) and water/acetic.
Download figure:
Standard image High-resolution image3.3. FTIR analysis
Knitted cotton fabrics, Jersey 20/1  , were used for all types of treatments assessed for FTIR analysis. Typically, cellulosic materials show two main absorbance regions in IR spectrum. The first one at low wavenumbers in the range
, were used for all types of treatments assessed for FTIR analysis. Typically, cellulosic materials show two main absorbance regions in IR spectrum. The first one at low wavenumbers in the range  , and the second one at higher wavenumbers in range
, and the second one at higher wavenumbers in range  .
.
Figure 3(a) shows the FTIR spectra of untreated cotton fabric, cotton fabric treated with ZnO-NPs, ZnO-NPs/softener and ZnO-NPs/ citric acid. In general, data showed a broad peak at  is owing to the Zn-O stretching in all samples. Samples treated with ZnO-NPs associate with citric acid showed a broad shoulder at
is owing to the Zn-O stretching in all samples. Samples treated with ZnO-NPs associate with citric acid showed a broad shoulder at  representing the carbonyl groups of citric acid [21] and demonstrating the fixation of citric acid on the cotton fabrics.
representing the carbonyl groups of citric acid [21] and demonstrating the fixation of citric acid on the cotton fabrics.
Figure 3. (a) FTIR spectra of untreated and treated knitted cotton fabric with ZnO-NPs 10 g l−1 solution, Jersey 20/1  cotton fabric treated with ZnO-NPs/softener and ZnO-NPs/citric acid. ZnO-NPs emulsion was homogenized for 30 min with other additives and sonicated for 1 h before application on fabrics. FTIR spectra; (b) of untreated and treated knitted cotton fabric, Jersey 20/1
cotton fabric treated with ZnO-NPs/softener and ZnO-NPs/citric acid. ZnO-NPs emulsion was homogenized for 30 min with other additives and sonicated for 1 h before application on fabrics. FTIR spectra; (b) of untreated and treated knitted cotton fabric, Jersey 20/1  , with TiO2-NPs 10 g l−1 solution, cotton fabric treated with TiO2-NPs/softener and TiO2-NPs/citric acid. TiO2-NPs emulsion was homogenized for 30 min with other additives and sonicated for 1 h before application on fabrics.
, with TiO2-NPs 10 g l−1 solution, cotton fabric treated with TiO2-NPs/softener and TiO2-NPs/citric acid. TiO2-NPs emulsion was homogenized for 30 min with other additives and sonicated for 1 h before application on fabrics.
Download figure:
Standard image High-resolution imageFTIR spectra of treated fabrics with TiO2-NPs showed (figure 3(b)) no significant changes in the region  for the treated samples. On the contrary, new peaks were observed in the region of
for the treated samples. On the contrary, new peaks were observed in the region of  assigned for Ti-O stretching band and at
assigned for Ti-O stretching band and at  representing the coordination interaction between Ti and OHs groups of glucose units [22–24].
representing the coordination interaction between Ti and OHs groups of glucose units [22–24].
Samples treated with TiO2-NPs associate with citric acid showed a broad shoulder at  representing the carbonyl groups of citric acid and demonstrating the fixation of citric acid on the cotton fabrics [21].
representing the carbonyl groups of citric acid and demonstrating the fixation of citric acid on the cotton fabrics [21].
3.4. Atomic absorption
The total content of metal oxides in the treated fabrics was quantitatively determined by the flam atomic absorption.
Tables 1 and 2 show the Zn and Ti content in percentage per 1 g fabrics of un/dyed samples treated with ZnO-NPs and TiO2-NPs (2.5, 5 and 10 g l−1) with/without cross-linker. Cotton fabric specifications were as follow: A: Milton  ; B: Peque
; B: Peque  ; C: Jersey 20/1
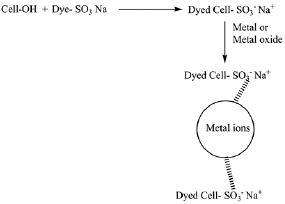
; C: Jersey 20/1  . The data show that the better binding efficiency was recorded to samples treated with softener over the citric acid treated samples. However, difference between ZnO and TiO2 in binding to the dyed fabric was investigated representing the ability of ZnO-NPs to bind to the negative charge of the dyed fabrics better than TiO2-NPs. TiO2-NPs have partly negative charge of the oxygen lone pairs (scheme 1) which may attribute to reduction of lower Ti content for dyed samples. Also, data showed that metal oxides uptake to the dyed samples is much higher than the undyed samples. This is back to charge attraction between metals and negative charges of dye molecules as shown in scheme 2.
. The data show that the better binding efficiency was recorded to samples treated with softener over the citric acid treated samples. However, difference between ZnO and TiO2 in binding to the dyed fabric was investigated representing the ability of ZnO-NPs to bind to the negative charge of the dyed fabrics better than TiO2-NPs. TiO2-NPs have partly negative charge of the oxygen lone pairs (scheme 1) which may attribute to reduction of lower Ti content for dyed samples. Also, data showed that metal oxides uptake to the dyed samples is much higher than the undyed samples. This is back to charge attraction between metals and negative charges of dye molecules as shown in scheme 2.
Table 1. ZnO-NPs content in percentage per 1 g fabric of un/dyed samples treated with ZnO-NPs (different concentrations) with/without cross-linker. Cotton fabric specifications: A: Milton  ; B: Peque
; B: Peque  ; C: Jersey 20/1
; C: Jersey 20/1  .
.
| No. | Samples | ZnO-NPs Conc. | Zn content (%) | |||
|---|---|---|---|---|---|---|
| g l−1 | A | B | C | |||
| Black | 0.00 | 0.00 | 0.00 | |||
| 1 | White Fabrics | Samples treated with ZnO -NPs with softener | 2.5 | 0.14 | 0.13 | 0.11 |
| 2 | 5 | 0.31 | 0.20 | 0.26 | ||
| 3 | 10 | 0.49 | 0.25 | 0.33 | ||
| 4 | Samples treated with ZnO -NPs with citric acid | 2.5 | 0.18 | 0.12 | 0.10 | |
| 5 | 5 | 0.24 | 0.17 | 0.22 | ||
| 6 | 10 | 0.35 | 0.27 | 0.31 | ||
| 7 | Samples treated with ZnO-NPs | 10 | 0.40 | 0.37 | 0.39 | |
| 8 | Dyed fabrics | Samples treated with ZnO-NPs with softener | 5 | 0.33 | 0.28 | 0.30 |
| 9 | Samples treated with ZnO-NPs with softener | 10 | 0.49 | 0.44 | 0.35 | |
| 10 | Samples treated with ZnO-NPs with citric acid | 5 | 0.27 | 0.21 | 0.18 | |
| 11 | Samples treated with ZnO-NPs with citric acid | 10 | 0.38 | 0.32 | 0.36 | |
Table 2. TiO2-NPs content in percentage per 1 g fabric of un/dyed samples treated with TiO2-NPs (different concentrations) with/without cross-linker. Cotton fabric specs.: A: Milton  ; B: Peque
; B: Peque  ; C: Jersey 20/1
; C: Jersey 20/1  .
.
| No | Samples | TiO2-NPs Conc. | Ti content (%) | |||
|---|---|---|---|---|---|---|
| g l−1 | A | B | C | |||
| Black | 0.00 | 0.00 | 0.00 | |||
| 1 | White fabrics | Samples treated with TiO2-NPs with softener | 2.5 | 0.25 | 0.20 | 0.18 |
| 2 | 5 | 0.47 | 0.41 | 0.44 | ||
| 3 | 10 | 0.73 | 0.61 | 0.48 | ||
| 4 | Samples treated with TiO2-NPs with citric acid | 2.5 | 0.22 | 0.19 | 0.20 | |
| 5 | 5 | 0.44 | 0.36 | 0.36 | ||
| 6 | 10 | 0.76 | 0.53 | 0.58 | ||
| 7 | Samples treated with TiO2-NPs | 10 | 0.58 | 0.49 | 0.52 | |
| 8 | Dyed fabrics | Samples treated with TiO2-NPs with softener | 5 | 0.35 | 0.33 | 0.23 |
| 9 | Samples treated with TiO2-NPs with softener | 10 | 0.49 | 0.45 | 0.35 | |
| 10 | Samples treated with TiO2-NPs with citric acid | 5 | 0.34 | 0.27 | 0.27 | |
| 11 | Samples treated with TiO2-NPs with citric acid | 10 | 0.39 | 0.37 | 0.33 | |
Scheme 1. Titanium dioxide chemical structure.
Download figure:
Standard image High-resolution imageScheme 2. The proposed mechanism of loading nano-metal and the metal oxides onto the dyed cellulosic substrates.
Download figure:
Standard image High-resolution image3.5. Self-cleaning
The self-cleaning potential of un-treated and treated white cotton fabrics were examined by the change in the color strength of the methylene blue (MB) before and after sunlight exposure [25, 26]. It is well known that titanium dioxide shows self-cleaning activities due to its ability to produce free radicals as a result of exposure to light photons. Such active species (e.g. free radicals) play the key role in color fading by converting the unsaturated chromophore-based systems, in colored compounds, to saturated system with no color. Same action has been recorded for ZnO-NPs treated fabric as well. This phenomenon is known as dye decomposition [27].
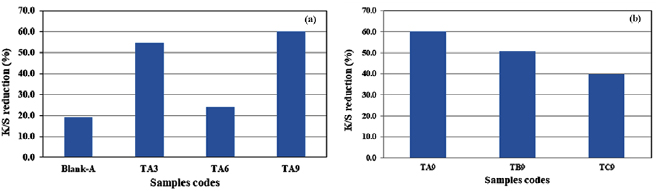
Figure 4(a) shows the K/S values of the un/treated white cotton fabrics with TiO2 with and without different fixation additives. All samples were exposed to sunlight for 24 h and K/S values were recorded before and after exposure. Data showed that the amount of discoloration of the treated samples is higher than that of untreated samples. Precisely, samples treated with TiO2 without any additives for fixation showed higher discoloration than those treated with either citric acid (TA6) or with reactive softener (TA3). This is may be attributed to that citric acid and the reactive softener increases the dye uptake of the basic dye more than the untreated samples.
Figure 4. (a) K/S reduction values for treated fabrics with TiO2 before and after sunlight exposure as a function of different fixing agents. Blank-A: Milton cotton fabric  ; TA3: Milton fabric treated with 10 g l−1 of TiO2 with softener; TA6: Milton fabric treated with 10 g l−1 of TiO2 with citric acid; TA9: Milton fabric treated with 10 g l−1 TiO2. (b) K/S reduction for treated fabrics with TiO2 before and after sunlight exposure as a function of different weaving structures of fabric. TA: Milton cotton fabric
; TA3: Milton fabric treated with 10 g l−1 of TiO2 with softener; TA6: Milton fabric treated with 10 g l−1 of TiO2 with citric acid; TA9: Milton fabric treated with 10 g l−1 TiO2. (b) K/S reduction for treated fabrics with TiO2 before and after sunlight exposure as a function of different weaving structures of fabric. TA: Milton cotton fabric  ; TB: Peque
; TB: Peque  ; TC: Jersey 20/1
; TC: Jersey 20/1  . All samples were treated with 10 g l−1 TiO2 without any additives.
. All samples were treated with 10 g l−1 TiO2 without any additives.
Download figure:
Standard image High-resolution imageFigure 4(b) shows the K/S reduction values for different fabric knitting structures [TA: Milton cotton fabric  ; TB: Peque
; TB: Peque  ; TC: Jersey 20/1
; TC: Jersey 20/1  ] treated with 10 g l−1 with wet-pick up 80%, dried at 120 °C for 5 min and thermo-cured at 160 °C for 3 min.
] treated with 10 g l−1 with wet-pick up 80%, dried at 120 °C for 5 min and thermo-cured at 160 °C for 3 min.
It is clear from data that the heavier the fabric the higher values in dye discoloration. In other words, treated fabric As [Milton cotton fabric  ] showed highest value in dye discoloration while treated fabric Cs showed the lowest values. This is attributed to the amount of the TiO2-NP impregnated into the fabric and as the heavier fabrics (As) contains more the efficiency of the color decomposition increased.
] showed highest value in dye discoloration while treated fabric Cs showed the lowest values. This is attributed to the amount of the TiO2-NP impregnated into the fabric and as the heavier fabrics (As) contains more the efficiency of the color decomposition increased.
Figure 5 shows the difference between fabrics treated with TiO2-NPs and ZnO-NPs. Data showed that dye discoloration values are almost identical for samples treated with softeners. However, with additive-free treatments (C9s) TiO2-NPs treated fabrics showed superior color fading (dye decomposition) over the ZnO-NPs treated ones [27]. This is attributed to the accessibility of the generated free-radicals is higher in the additive-free samples to reach the dye molecules and decompose chromophores.
Figure 5. K/S reduction values for TiO2-NPs treated fabrics (TA3, TA6 and TA9) and the ZnO-NPs treated fabrics (ZA3, ZA6 and ZA9). Samples were treated in 10 g l−1 metal oxide (TA9 and ZA9) along with softener (TA3 and ZA3) or citric acid (TA6 and ZA6).
Download figure:
Standard image High-resolution image3.6. Antibacterial activity
The antibacterial activities of the treated samples were quantitatively demonstrated by using gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria.
Samples treated with ZnO-NPs and TiO2-NPs emulsions with/without fixing agents were sterilized by autoclaving at 120 °C for 15 min. One hundred mg of each sample was tested in triplicate against each bacterial strain. Each sample was tested in triplicate in weight of 100 mg to each strain and the untreated cotton fabric was used as control. Development of the visible turbidity after 24 h of incubation is referring to growth of the microorganisms and inactivity of the treatment. In contrast, clear nutrient broth with no visible turbidity is revealing suppression of the microbial growth and antimicrobial effect of the tested treatment (figure 6). For further evaluation, bacterial counting for different samples was performed to identify the antimicrobial effect if it is inhibition or cidal.
Figure 6. A 24-well plate showing turbidity development in different ZnO-NPs treatments samples inoculated with E.coli after shaking incubation for 24 h at 37 °C.
Download figure:
Standard image High-resolution image3.7. Antibacterial assessment of Zinc oxide nanoparticles treated samples
Inorganic metal oxides in nano form such as ZnO-NPs have been shown a promising antimicrobial activity without harmful effect. ZnO-NPs is considered as stable at high temperature and safe to human health. It has been documented [28, 29] that the antimicrobial activity of ZnO-NPs against both types of bacteria (gram positive and gram negative) is back to their photo-catalytic activity. This leads to subsequent generation of reactive oxygen species such as ·OH,  and
and  in the presence of light and water and this causes inhibition of cell manipulation and consequently cell death. This along with, the abrasion effects of the nanoparticles of ZnO upon the cell membrane leading to Zn2+ release which intensify the antibacterial functionalization [3].
in the presence of light and water and this causes inhibition of cell manipulation and consequently cell death. This along with, the abrasion effects of the nanoparticles of ZnO upon the cell membrane leading to Zn2+ release which intensify the antibacterial functionalization [3].
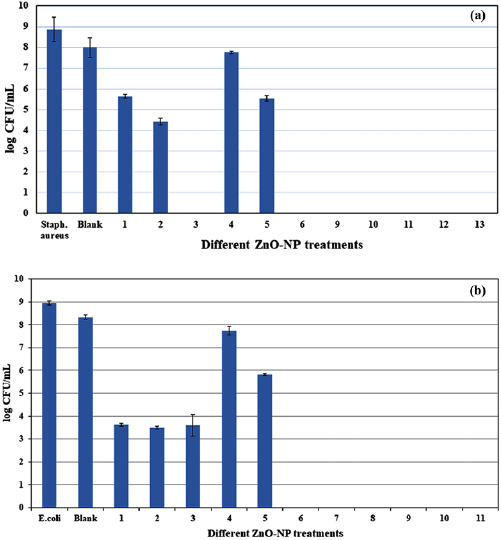
Figures 7(a) and (b) show the mean counts of both S. aureus and E. coli bacteria respectively for dyed and white samples treated by ZnO-NPs emulsions containing either reactive softener or citric acid.
Figure 7. (a) Average of viable S. aureus count, Colony forming units, (CFU). Blank: untreated fabric; 1: cotton fabric treated by 2.5 g l−1 ZnO with softener; 2: cotton fabric treated by 5 g l−1 ZnO with softener; 3: cotton fabric treated with 10 g l−1 ZnO with softener; 4: cotton fabric treated with 2.5 g l−1 ZnO with citric acid; 5: cotton fabric treated with 5 g l−1 ZnO with citric acid; 6: cotton fabric treated with 10 g l−1 ZnO with citric acid; 7: cotton fabric treated with 10 g l−1; 8: dyed cotton fabric treated by 5 g l−1 ZnO with softener; 9: dyed cotton fabric treated by 10 g l−1 ZnO with softener; 10: dyed cotton fabric treated by 5 g l−1 ZnO with citric acid; 11: dyed cotton fabric treated by 10 g l−1 ZnO with citric acid; (b) Average of viable E. coli count, Colony forming units, (CFU). Blank: untreated fabric; 1: cotton fabric treated by 2.5 g l−1 ZnO with softener; 2: cotton fabric treated by 5 g l−1 ZnO with softener; 3: cotton fabric treated with 10 g l−1 ZnO with softener; 4: cotton fabric treated with 2.5 g l−1 ZnO with citric acid; 5: cotton fabric treated with 5 g l−1 ZnO with citric acid; 6: cotton fabric treated with 10 g l−1 ZnO with citric acid; 7: cotton fabric treated with 10 g l−1; 8: dyed cotton fabric treated by 5 g l−1 ZnO with softener; 9: dyed cotton fabric treated by 10 g l−1 ZnO with softener; 10: dyed cotton fabric treated by 5 g l−1 ZnO with citric acid; 11: dyed cotton fabric treated by 10 g l−1 ZnO with citric acid.
Download figure:
Standard image High-resolution imageAs shown in figures 7(a) and (b), treatments with ZnO-NPs (10 g l−1) have bateriocidal effect lead to complete reduction of both Gram negative and positive bacteria. However, dyed samples showed higher bacteria reduction at lower concentration of ZnO-NPs (5 g l−1). Such phenomenon is due to the capability of ZnO-NPs to generate some active species of oxy-radicals in the solution [30]. This active species react with bacteria membrane and suppressed their replications.
Figures 8(a) and (b) show the mean counts of three replicate of both S. aureus and E. coli bacteria respectively for dyed and undyed samples treated by TiO2-NPs emulsions containing either reactive softener or citric acid. Data shows very weak inhibition ability of the treated samples against both S. aureus and E. coli bacteria. Of the tested samples, dyed cotton fabric treated with 5 g l−1 TiO2-NPs in the presence of softener showed an inhibition effect against only S. aureus where the counting for this treatment was almost equal to the initial inoculation  . In general, the antibacterial activity of TiO2 relies on the active species formed in the presence of UV irradiation [10]. However, during the antibacterial assay, procedure was conducted in dark environment. Therefore, results showed limited reduction throughout the treated fabrics.
. In general, the antibacterial activity of TiO2 relies on the active species formed in the presence of UV irradiation [10]. However, during the antibacterial assay, procedure was conducted in dark environment. Therefore, results showed limited reduction throughout the treated fabrics.
Figure 8. (a) Average of viable S. aureus count, Colony forming units, (CFU). Blank: untreated fabric; 1: cotton fabric treated by 2.5 g l−1 TiO2-NPs with softener; 2: cotton fabric treated by 5 g l−1 TiO2-NPs with softener; 3: cotton fabric treated with 10 g l−1 TiO2-NPs with softener; 4: cotton fabric treated with 2.5 g l−1 TiO2-NPs with citric acid; 5: cotton fabric treated with 5 g l−1 TiO2-NPs with citric acid; 6: cotton fabric treated with 10 g l−1 TiO2-NPs with citric acid; 7: cotton fabric treated with 10 g l−1; 8: dyed cotton fabric treated by 5 g l−1 TiO2-NPs with softener; 9: dyed cotton fabric treated by 10 g l−1 TiO2-NPs with softener; 10: dyed cotton fabric treated by 5 g l−1 TiO2-NPs with citric acid; 11: dyed cotton fabric treated by 10 g l−1 TiO2-NPs with citric acid; (b) Average of viable E. coli count, Colony forming units, (CFU). Blank: untreated fabric; 1: cotton fabric treated by 2.5 g l−1 TiO2-NPs with softener; 2: cotton fabric treated by 5 g l−1 TiO2-NPs with softener; 3: cotton fabric treated with 10 g l−1 TiO2-NPs with softener; 4: cotton fabric treated with 2.5 g l−1 TiO2-NPs with citric acid; 5: cotton fabric treated with 5 g l−1 TiO2-NPs with citric acid; 6: cotton fabric treated with 10 g l−1 TiO2-NPs with citric acid; 7: cotton fabric treated with 10 g l−1; 8: dyed cotton fabric treated by 5 g l−1 TiO2-NPs with softener; 9: dyed cotton fabric treated by 10 g l−1 TiO2-NPs with softener; 10: dyed cotton fabric treated by 5 g l−1 TiO2-NPs with citric acid; 11: dyed cotton fabric treated by 10 g l−1 TiO2-NPs with citric acid.
Download figure:
Standard image High-resolution image3.8. UV-protection
Table 3 shows the UPF values and Zn content of the un/dyed samples treated with ZnO-NPs (different concentrations) with either reactive softener or citric acid as fixing agent.
Table 3. UPF values of un/dyed samples treated with ZnO-NPs (different concentrations) with/without cross-linker. Cotton fabric specs.: A: Milton  ; B: Peque
; B: Peque  ; C: Jersey 20/1
; C: Jersey 20/1  .
.
| No | Sample | ZnO-NPs conc. | UPF values | |||
|---|---|---|---|---|---|---|
| g/l | % | A | B | C | ||
| Black | 51.64 | 11.53 | 10.18 | |||
| 1 | Samples treated with ZnO-NPs with softener | 2.5 | 0.11 | 25 | ||
| 2 | 5 | 0.26 | 45 | |||
| 3 | 10 | 0.33 | 338 | 46.85 | 88 | |
| 4 | Samples treated with ZnO-NPs with citric acid | 2.5 | 0.10 | 19 | ||
| 5 | 5 | 0.22 | 40 | |||
| 6 | 10 | 0.31 | 743 | 12.32 | 75 | |
| 7 | Samples treated with ZnO-NPs | 10 | 0.39 | 464 | 131 | 113 |
| 8 | Dyed samples treated with ZnO-NPs with softener | 5 | 0.30 | 702 | ||
| 9 | Dyed samples treated with ZnO-NPs with softener | 10 | 0.35 | 2610 | 1446 | 753 |
| 10 | Dyed samples treated with ZnO-NPs with citric acid | 5 | 0.18 | 424 | ||
| 11 | Dyed samples treated with ZnO-NPs with citric acid | 10 | 0.36 | 1386 | 454 | 434 |
Data showed that samples treated with ZnO-NPs are in agreement with Zn content. The higher Zn content the better UPF values as compared to the untreated sample. Results also showed that samples treated with softener are recording higher values than those treated with citric acid. In general, UPF values increase as the ZnO-NPs pick-up concentration increases. However, samples treated with citric acid showed lower UPF values lower than those treated with the reactive softener. It is notable that the highest UPF values were recorded for the dyed samples throughout the whole samples. This phenomenon is due to the ability of the dye molecules to absorb the harmful UV-radiation.
It has been reported [31] that UPF limits of the treated garment for UV-protection should be at least 40–50+. Thus, samples treated with 10 g l−1 ZnO-NPs associated with excellent UV protection. Regarding to TiO2-NPs treated samples, data showed that discoloration values are almost identical for samples treated with softeners. However, with additive-free treatments TiO2-NPs treated fabrics showed superior color fading (dye decomposition) over the ZnO-NPs treated ones [27]. This is attributed to the accessibility of the generated free-radicals is higher in the additive-free samples to reach the dye molecules and decomposing chromophores.
4. Conclusion
ZnO and TiO2 are prepared in nano-sized particles of 3–5 nm and 8–15 nm, respectively, from their precursors and characterized to confirm their crystal forms. Knitted cotton fabrics are treated by aqueous solutions of ZnO-NPs and TiO2-NPs with/without fixing agents. Treated fabrics with ZnO-NPs showed antimicrobial activities against gram-positive and gram-negative better than those treated with TiO2-NPs. On contrary, samples treated with TiO2-NPs showed superior values for self-cleaning ability over those treated with ZnO-NPs. The UPF values of the samples treated with TiO2-NPs showed better UV-protection than untreated fabrics. 5 g l−1 of TiO2-NPs are able to show UPF vales over 50+. However, samples treated with 10 g l−1 ZnO-NPs associated with excellent UV protection.
Acknowledgments
This project was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant No: 9176). Authors are grateful to National Research Centre for facilities provided. The authors have declared no conflicts of interest.