Abstract
The aim of this study is to investigate the bacterial adherence on 5, 10 and 15 nm nanosilver-impregnated resorbable guided tissue regeneration (GTR) membranes. GTR membranes were incorporated with 5 (NS-GTR-5), 10 (NS-GTR-10), and 15 nm (NS-GTR-15) nanosilver in the test group. The control group contained GTR membranes incorporated with 25% metronidazole (M-GTR) and plain membranes (GTR-C). Qualitative evaluation of microbial adherence to the GTR membranes was performed using four organisms-Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Porphyromonas gingivalis, and Streptococcus mutans-since these organisms are reported to have strong adherent capabilities to collagen membranes. The main results have been obtained: The mean bacterial adherence scores were significantly greater  in the GTR-C group when compared to those in the M-GTR and NS-GTR-5, NS-GTR-10, and NS-GTR-15 groups. M-GTR showed higher adherence scores than NS-GTR-5, NS-GTR-10, and NS-GTR-15 for all microorganisms; the differences were statistically significant. We have concluded that collagen membranes impregnated with 5- and 15 nm nanosilver exhibited an apparent antibacterial effect against anaerobic oral pathogenic bacteria and aerobic bacteria. This outcome indicates that nanosilver-impregnated GTR membranes may be useful in periodontal regeneration therapy.
in the GTR-C group when compared to those in the M-GTR and NS-GTR-5, NS-GTR-10, and NS-GTR-15 groups. M-GTR showed higher adherence scores than NS-GTR-5, NS-GTR-10, and NS-GTR-15 for all microorganisms; the differences were statistically significant. We have concluded that collagen membranes impregnated with 5- and 15 nm nanosilver exhibited an apparent antibacterial effect against anaerobic oral pathogenic bacteria and aerobic bacteria. This outcome indicates that nanosilver-impregnated GTR membranes may be useful in periodontal regeneration therapy.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Periodontitis is a worldwide prevalent inflammatory condition that leads to progressive destruction of periodontal tissues, specifically the alveolar bone, periodontal ligaments, and root cementum, and is the key cause of tooth loss in adults. Periodontal regeneration is defined as the reconstruction or restoring of lost or injured tissue such that the function and form of the lost structures are restored. To date, flap debridement and periodontal regenerative therapy with membranes and bone grafting materials have been employed with distinct levels of clinical success. Guided tissue regeneration (GTR) has been established as a reliable method to regain the lost periodontal attachment around teeth, repair the bone dehiscence associated with dental implants, and augment resorbed ridges [1–3]. A variety of novel resorbable and non-resorbable biomaterials are being used clinically or are being tested experimentally for use with GTR procedures. The GTR technique employs a resorbable barrier membrane around the periodontal defect to avoid epithelial downgrowth and to promote fibroblast transgrowth into the wound space, which in turn maintains a space for true periodontal tissue regeneration. Although the validity of this biological principle has been established in a large number of animal models [4–6], human trials have shown poor overall predictability of GTR procedures [7–9]. Bacterial contamination represents the most significant factor leading to a compromised outcome in periodontal regeneration using GTR technique. Microbial phenotypes, the bacterial mass, and the extent of bacterial contamination on the GTR membrane are some the factors affecting the outcome of GTR [10]. The bacterial loads on GTR membranes include various gram-positive bacteria as well as periodontal pathogens. The bacterial species count existing on the membrane is positively related with gingival recession and negatively associated with clinical attachment gain [11]. Presently, there are limited data on the qualitative or quantitative characteristics of the microbiota that adhere to exposed membranes [12]. Therefore, integration of an antibiotic into the membrane may help control membrane-associated infection during GTR therapy [13, 14]. Recent studies have suggested that doxycycline-loaded GTR membranes may have a beneficial effect on osteogenesis and favour periodontal regeneration [15, 16].
In the last few decades, much attention has been paid to antimicrobial polymers and their nanostructures due to their comprehensive applications in dentistry and animal healthcare [17–19]. These polymers usually form secondary structures to enrich antibacterial groups and thereby improve drug deliverability. Thus, they can kill bacteria upon contact and show durable and sustainable antimicrobial activity when bacteria covalently attach to these surfaces [20]. Silver nanoparticles are some of the most broadly commercialized nanomaterials used in clinical care and consumer products; these nanoparticles have shown great toxicity to a broad range of microorganisms and can effectively kill both gram-negative and gram-positive bacteria [21, 22]. As a naturally antibacterial metal, a silver nanoparticle has multiple mechanisms of antibacterial activity. First, the presence of a large number of nanoparticles inside the bacteria is thought to affect the membrane permeability of bacteria. The interaction of silver nanoparticles with the bacterial membrane and intracellular proteins, particularly the sulphur-containing membrane proteins and phosphorus-containing DNA, interferes with cell division and causes cell death. The antibacterial activity of a material is related to many factors, such as the formulation effects, presence of an organic load, synergy, temperature, and dilution [23]. To improve this antibacterial activity, a great volume of work in the literature has focused on designing the ideal antibacterial nanoparticles. Overall, the particles' synthetic chemistry, morphology, size, and surface charge are among the most appropriate variables affecting antibacterial activity [24, 25]. A recent study reported that nanosilver impregnated into chitosan membranes has the capability to control suspected periodontopathic bacteria [26–28]. Thus, the present in vitro study was designed to evaluate the bacterial adherence on 5-, 10-, and 15 nm nanosilver-impregnated resorbable GTR membranes.
2. Materials and method
2.1. Bacterial and culture conditions
The bacterial strains selected and used in the present study were Aggregatibacter actinomycetemcomitans (ATCC 29523), Fusobacterium nucleatum (ATCC 25586), Porphyromonas gingivalis (ATCC 33277), and Streptococcus mutans (ATCC 25175). A. actinomycetemcomitans was cultured in brain heart infusion (BHI) agar/broth at  and incubated in an anaerobic environment at an atmosphere of 75%
and incubated in an anaerobic environment at an atmosphere of 75%  , 5%
, 5%  , and 20%
, and 20%  at
at  . F. nucleatum was cultured in modified chopped meat medium and P. gingivalis was cultured in supplemented tryptic soy broth/agar; both were incubated in an anaerobic environment with an atmosphere of 80%
. F. nucleatum was cultured in modified chopped meat medium and P. gingivalis was cultured in supplemented tryptic soy broth/agar; both were incubated in an anaerobic environment with an atmosphere of 80%  , 10%
, 10%  , and 10%
, and 10%  at
at  . S. mutans was cultured in BHI agar/broth in anaerobic conditions, incubated at
. S. mutans was cultured in BHI agar/broth in anaerobic conditions, incubated at  for 24 to 48 h. All these four strains were reported to strongly adhere to GTR membranes composed of materials such as collagen, polytetrafluoroethylene and polylactic acid blended with citric acid ester (PLA) [29].
for 24 to 48 h. All these four strains were reported to strongly adhere to GTR membranes composed of materials such as collagen, polytetrafluoroethylene and polylactic acid blended with citric acid ester (PLA) [29].
2.2. Sample size estimation
The sample size was calculated according to the formula  where
where  is the sample size,
is the sample size,  is the value from the normal distribution table,
is the value from the normal distribution table,  is the detection level and
is the detection level and  is the standard deviation from the pilot data. To detect a
is the standard deviation from the pilot data. To detect a  difference in inner tube cultures, a minimum of 14 membranes per organism per time frame were essential, assuming
difference in inner tube cultures, a minimum of 14 membranes per organism per time frame were essential, assuming  ,
,  from pilot culture studies, and
from pilot culture studies, and  . In the present study, 20 membranes per organism per time interval were used.
. In the present study, 20 membranes per organism per time interval were used.
2.3. Structure, morphology and physical properties of GTR membranes
GTR membranes had an irregular side surface with numerous spherical depressions arranged homogeneously throughout its extension. The membrane thickness was uniform and measured approximately 0.65 mm. The outer surface seemed to be very smooth and homogeneous. The inner surface exhibited numerous fibers of varying thicknesses, arranged in several directions, forming an interconnected mesh with retentive regions. The tensile strength of membranes was ranging from 12.4 to 13.0 Mpa.
2.4. Fabrication of nanosilver-loaded GTR membranes (test groups)
Collagen membranes were selected to fabricate the nanosilver-impregnated GTR membranes. Membranes were cut into 6 mm-diameter collagen discs in a sterile environment. Silver nanoparticles in a colloidal solution with concentration gradients of 0.05 mg ml−1 (PlasmaChem GmbH, Berlin, Germany; 100 ml; average particle size: 5 nm), 0.1 mg ml−1 (PlasmaChem GmbH, Berlin, Germany; 100 ml; average particle size: 10 nm), and 0.15 mg ml−1 (PlasmaChem GmbH, Berlin, Germany; 100 ml; average particle size: 15 nm) in water were utilized to prepare nanosilver-impregnated GTR membranes of 5 nm (NS-GTR-5), 10 nm (NS-GTR-10), and 15 nm (NS-GTR-15), respectively. Silver nanoparticles were applied by immersion of the collagen membranes in the colloidal solution for 3 h at  .
.
2.5. Fabrication of metronidazole-loaded GTR membranes (control group)
A readymade solution of 100 mg ml−1 metronidazole (Sigma-Aldrich, Hyderabad, India) was reduced to a concentration of 25%, and the membrane was coated with this solution by immersion for 3 h to prepare the metronidazole-impregnated GTR membranes (MET-GTR) [15]. Plain GTR membranes (GTR-C) were used as positive controls.
The primed membranes were freeze-dried in a commercially available laboratory freeze dryer (Lyophilization Systems Pvt Ltd, Hyderabad, India) as per the technique proposed by Li et al [30]. This treatment provides the membranes light to dark yellowish hues upon finishing point. Freeze drying preserves the physical structure and maintains the integrity of the material for storage and transport. At the same time, freeze-dried materials can be re-formed quickly and easily, without negotiating the original physical or biological properties of the material [31].
2.6. Qualitative observation of bacterial adhesion on GTR membranes
A microscopic counting technique was used to measure fluorescent-tagged bacteria and thereby determine the qualitative bacterial adhesion on GTR membranes [13, 32].
2.7. Statistical analysis
Statistical analysis was performed using the statistical package for social sciences (SPSS) version 24 (IBM. Inc, Chicago). Descriptive statistics included the mean and standard deviation for bacterial adherence on various membranes. Two-way analysis of variance (ANOVA) repeated-measures test was employed to test the differences for multiple means. Significant difference among various membranes was evaluated by ANOVA test that influenced the level of bacterial adherence. The analysis was considered to test differences of the mean bacterial adherence among the types of bacteria on each membrane and to test differences in the mean bacteria adherence among the membranes of each type of bacteria. Differences were considered significant when the ANOVA value of the Bonferroni adjusted t-test was <0.05. Scheffe test was used for post-hoc analysis.
3. Results and discussion
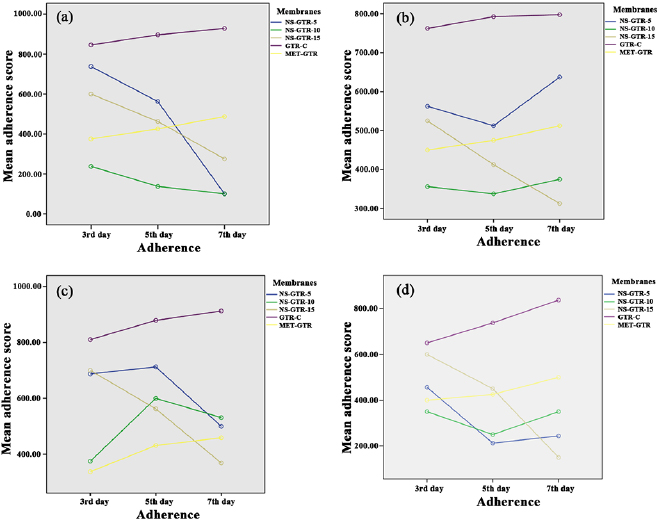
Nineteen membranes were used per organism for each time interval in the five groups for evaluating microbial adherence. The microbial adherence of the four selected microorganisms in their respective membrane groups were evaluated over three time intervals. The mean adherence scores assessed by colony-counting assays for the four microorganisms in the five membrane groups are presented in table 1. The adherence scores of F. nucleatum decreased at the end of day 7 in groups NS-GTR-5, NS-GTR-10, and NS-GTR-15 and increased in groups GTR-C and MET-GTR (figure 1(a)). The adherence scores for P. gingivalis increased in all groups except the NS-GTR-15 group (figure 1(b)). The mean adherence scores for S. mutans varied in groups, with decreasing scores were observed in the NS-GTR-5 and NS-GTR-10 groups (figure 1(c)). In the GTR-C group, the mean adherence scores for all microorganisms showed an increase at the end of days 3, 5, and 7, whereas the mean adherence scores for A. actinomycetemcomitans decreased in groups NS-GTR-15 and NS-GTR-5 (figure 1(d)).
Table 1. Mean adherence scores of bacteria on membranes evaluated at days 3, 5, and 7.
| Bacteria | Membranes 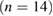 |
Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|
| F. nucleatum | NS-GTR-5 | 737.5 (±47.5) | 562.5 (±47.8) | 100.0 (±23.9) |
| NS-GTR-10 | 237.5 (±57.3) | 137.5 (±68.3) | 100.0 (±40.8) | |
| NS-GTR-15 | 600 (±17.48) | 462.5 (±28.8) | 275.0 (±28.8) | |
| GTR-C | 846.2 (±101.1) | 896.2 (±53.7) | 928.7 (±36.1) | |
| MET-GTR | 375 (±28.8) | 425.0 (±28.8) | 487.5 (±25.8) | |
| P. gingivalis | NS-GTR-5 | 562.5 (±47.8) | 512.5 (±62.9) | 637.5 (±170.1) |
| NS-GTR-10 | 356.2 (±42.6) | 337.5 (±47.8) | 375.0 (±95.7) | |
| NS-GTR-15 | 525.0 (±95.7) | 412.5 (±131.4) | 312.5 (±103.0) | |
| GTR-C | 762.5 (±25) | 792.5 (±43.9) | 797.5 (±105.0) | |
| MET-GTR | 450.0 (±40.8) | 475.0 (±86.6) | 512.5 (±85.3) | |
| S. mutans | NS-GTR-5 | 687.5 (±85.9) | 712.5 (±85.3) | 500.0 (±.0) |
| NS-GTR-10 | 375.0 (±95.7) | 600.0 (±.0) | 531.2 (±21.7) | |
| NS-GTR-15 | 700.0 (±.0) | 562.5 (±47.8) | 368.7 (±23.9) | |
| GTR-C | 810.0 (±40.6) | 878.7 (±48.0) | 912.5 (±12.5) | |
| MET-GTR | 337.5 (±47.8) | 431.2 (±23.9) | 458.7 (±38.3) | |
| A. actinomycetemcomitans | NS-GTR-5 | 456.5 (±42.6) | 212.5 (±85.3) | 243.7 (±42.6) |
| NS-GTR-10 | 350.0 (±40.8) | 250.0 (±40.8) | 350.0 (±40.8) | |
| NS-GTR-15 | 600.0 (±.0) | 450.0 (±23.9) | 150.0 (±54.2) | |
| GTR-C | 650.0 (±108.1) | 737.5 (±103.0) | 837.5 (±62.9) | |
| MET-GTR | 400.0 (±10.8) | 425.0 (±64.5) | 500.0 (±108.0) | |
Figure 1. Mean membrane adherence score of (a) F. nucleatum; (b) P. gingivalis; (c) S. mutans; (d) A. actinomycetemcomitans.
Download figure:
Standard image High-resolution image3.1. Intragroup comparisons at days 3, 5, and 7
The decrease in the mean adherence score in the NS-GTR-5 group was statistically significant  whereas that in the NS-GTR-10 group was not significant. The decrease in the mean adherence score at the end of days 3, 5, and 7 in the NS-GTR-15 group was highly significant
whereas that in the NS-GTR-10 group was not significant. The decrease in the mean adherence score at the end of days 3, 5, and 7 in the NS-GTR-15 group was highly significant  for all four microorganisms. In the GTR-C and MET-GTR groups, the increase in mean adherence scores for all four microorganisms at the end of days 3, 5, and 7 was significant when compared to the scores at day 3
for all four microorganisms. In the GTR-C and MET-GTR groups, the increase in mean adherence scores for all four microorganisms at the end of days 3, 5, and 7 was significant when compared to the scores at day 3  (table 2).
(table 2).
Table 2. Membrane-wise comparison of bacterial adherence at days 3, 5, and 7.
| Membranes | 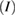 adherence adherence |
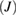 adherence adherence |
Mean difference 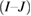 |
Std. error | Sig. |
|---|---|---|---|---|---|
| NS-GTR-5 | 1 | 2 | 110.938 |
25.011 | .000 |
| 3 | 240.625 |
38.319 | .000 | ||
| 2 | 1 | −110.938 |
25.011 | .000 | |
| 3 | 129.688 |
33.962 | .001 | ||
| 3 | 1 | −240.625 |
38.319 | .000 | |
| 2 | −129.688 |
33.962 | .001 | ||
| NS-GTR-10 | 1 | 2 | −1.563 | 25.011 | 1.000 |
| 3 | −9.375 | 38.319 | 1.000 | ||
| 2 | 1 | 1.563 | 25.011 | 1.000 | |
| 3 | −7.812 | 33.962 | 1.000 | ||
| 3 | 1 | 9.375 | 38.319 | 1.000 | |
| 2 | 7.812 | 33.962 | 1.000 | ||
| NS-GTR-15 | 1 | 2 | 134.375 |
25.011 | .000 |
| 3 | 329.688 |
38.319 | .000 | ||
| 2 | 1 | −134.375 |
25.011 | .000 | |
| 3 | 195.313 |
33.962 | .000 | ||
| 3 | 1 | −329.688 |
38.319 | .000 | |
| 2 | −195.313 |
33.962 | .000 | ||
| GTR-C | 1 | 2 | −59.063 | 25.011 | .062 |
| 3 | −101.875 |
38.319 | .029 | ||
| 2 | 1 | 59.063 | 25.011 | .062 | |
| 3 | −42.813 | 33.962 | .634 | ||
| 3 | 1 | 101.875 |
38.319 | .029 | |
| 2 | 42.813 | 33.962 | .634 | ||
| MET-GTR | 1 | 2 | −48.438 | 25.011 | .170 |
| 3 | −99.062 |
38.319 | .035 | ||
| 2 | 1 | 48.438 | 25.011 | .170 | |
| 3 | −50.625 | 33.962 | .421 | ||
| 3 | 1 | 99.062 |
38.319 | .035 | |
| 2 | 50.625 | 33.962 | .421 | ||
1, adherence score at day 3; 2, adherence score at day 5; 3, adherence score at day 7. aThe mean difference is significant at the .05 level.
Post-hoc comparisons revealed statistically significant  differences in microbial adhesion between the NS-GTR-5 group and the NS-GTR-10, GTR-C, and MET-GTR groups. The GTR-C group showed statistically significant
differences in microbial adhesion between the NS-GTR-5 group and the NS-GTR-10, GTR-C, and MET-GTR groups. The GTR-C group showed statistically significant  differences in comparison with all other groups since the mean bacterial adherence scores were greater in the GTR-C group with respect to all microorganisms across all intervals of time. The mean bacterial adherence in the NS-GTR group was lesser with respect to all bacteria; therefore, the NS-GTR-10 and GTR-C were the only two groups that showed statistically significant differences
differences in comparison with all other groups since the mean bacterial adherence scores were greater in the GTR-C group with respect to all microorganisms across all intervals of time. The mean bacterial adherence in the NS-GTR group was lesser with respect to all bacteria; therefore, the NS-GTR-10 and GTR-C were the only two groups that showed statistically significant differences  from the other contralateral groups. Although the mean bacterial adherence scores in the NS-GTR-15 group were lesser than those in the MET-GTR group across all time intervals and with respect all microorganisms, this difference in adherence scores was not statistically significant
from the other contralateral groups. Although the mean bacterial adherence scores in the NS-GTR-15 group were lesser than those in the MET-GTR group across all time intervals and with respect all microorganisms, this difference in adherence scores was not statistically significant  .
.
The aim of the current study was to evaluate bacterial adherence on drug-releasing GTR/guided bone regeneration (GBR) membranes with anti-infective capability and to evaluate their effects on bacterial growth and biocompatibility. The findings of a previous study suggested that bacterial adhesion decreases as the hydrophobicity of the biomaterial increases [33]. In this context, collagen membranes were used since these are more hydrophilic than other membranes and might help create an environment more suitable for bacterial growth. In the current study, bacterial adherence was found on all the membranes, including the test and control groups, under the experimental conditions as documented by Chen et al [29]. We hypothesize that nanosilver-impregnated resorbable membranes could reduce bacterial adherence and hence prevent infection, which is considered as the major reason for clinical failure of GTR/GBR membranes. In an article by Li, this was the reason for assessing resorbable membranes with different particle sizes of nanosilver [30]. Our study's primary findings indicated that nanosilver-impregnated GTR membranes had adequate antimicrobial activity to control the adhesion of microorganisms. This study focused on early bacterial adhesion, since it seems to be more important than secondary accumulation [34]. In the present study, MET-GTR membranes were used as positive controls because previous studies have emphasized that the clinical attachment gain of vertical periodontal defects after GTR is significantly favoured by the presence of metronidazole in the wound during the initial healing phase [35]. It was found that the collagen membranes impregnated with metronidazole released high levels of the drugs, at concentration well above the MIC values. The current study showed an apparent decrease in the levels of adhesion by S. mutans and A. actinomycetemcomitans onto the collagen membranes impregnated with 5-, 10-, and 15-nm nanosilver particles in comparison with the adhesion in the control group. Another study reported similar findings such as a decrease in adhesion of S. mutans and A. actinomycetemcomitans on antibiotic-loaded GTR membranes, which was mainly attributed to the antimicrobial effects of antibiotics [36]. Moreover, the mean adherence scores for F. nucleatum and S. mutans was found to be higher for plain collagen membranes. A similar in vitro study by Wang et al reported that the amounts of S. mutans or A. actinomycetemcomitans adhering onto collagen membranes were significantly higher than those adhering to ePTFE membranes [34]. The findings from the present study suggested that NS-GTR-5, NS-GTR-10, and NS-GTR-15 significantly inhibited the growth of four periodontal pathogenic bacteria, but more interestingly, silver nanoparticles of different sizes showed different rates and extent of bacterial adherence. NS-GTR-5 was more effective in reducing the adherence of F. nucleatum whereas NS-GTR-15 was more effective in reducing the adherence of P. gingivalis, S. mutans, and A. actinomycetemcomitans. These findings are in accordance with those reported by Zhong Lu et al, who observed that 5-nm Ag presents the highest antibacterial activity, with the MIC values of 5 nm Ag against the anaerobic oral pathogenic bacteria A. actinomycetemcomitans, F. nucleatum, S. mitis, S. mutans, and S. sanguis being 25, 25, 25, 50, and 50 g ml−1, respectively [37]. Another recent study suggested that GTR membranes incorporated with silver nanoparticles showed predictable antibacterial activity compared to doxycycline in controlling membrane-associated infection during GTR therapy [38].
Till date, the exact mechanism of action of silver nanoparticles on microbes is unknown, with possible mechanisms involving cell wall inhibition, formation of free radicals, attachment to the 30S subunit, inhibition of the thiol group of enzymes, prevention of biofilm formation, effects on the cell signalling molecules of bacterial peptides, and attachment to the surface of the cell wall [39]. Interactions of silver particles with the bacterial membrane and intracellular proteins, specifically sulphur-containing membrane proteins and phosphorus-containing DNA, inhibit the cell division, thus leading to cell death. Subsequently, few research studies have established the existence of biocidal ionic silver released from nanoparticle surfaces [40]. The bactericidal effect of silver nitrate particles is size-dependent, as the size of the silver nanoparticle indicates the surface area that will come in contact with the bacterial cell. Since NS-GTR-5 in the present study was the smallest with respect to particle size among the three samples, its contact with bacteria could be the largest. This could be the reason why NS-GTR-5 also presented the best antibacterial activity in terms of reduction of the mean bacterial adherence scores. Regarding the toxicity of nanosilver, in vitro and animal studies have shown that nanosilver is toxic, but its successful widespread use in wound dressings in burn cases has not revealed any toxicity in in vitro and animal studies [41].
Considering the results and recommendations from this study, one limitation of the study was that it could not establish the viability of the nanosilver antibacterial action till the enzymatic degradation of the membrane. Since the purpose of using these GTR membranes is space maintenance, this factor related to the availability is crucial. However, this study also provides the scope for future research evaluating the additive effect of copper chloride and zinc chloride on nanosilver-impregnated GTR membranes, since these salts have superior bactericidal action against periodontal pathogens. A deeper insight into the identification of more physiologically and biologically active GTR membranes with nanomaterials is essential to develop new approaches and materials that have broad and persistent microbe-killing capability and low toxicity to mammalian cells as well to promote periodontal regeneration. Despite there still being complaints about polymer membranes, such as their low mechanical properties, uncontrollable degradation speed and some other drawbacks, these problems will undoubtedly be conquered and biodegradable polymers will have more applications in GTR and GBR [36, 39, 42].
4. Conclusion
Incorporation of nanosilver onto collagen membranes significantly reduced the adhesion of S. mutans, A. actinomycetemcomitans, F. nucleatum, and P. gingivalis. Collagen membranes impregnated with 5 and 15 nm nanosilver exhibited apparent antibacterial effects against anaerobic oral pathogenic bacteria and aerobic bacteria. This outcome indicates nanosilver-impregnated GTR membranes can be effectively used for periodontal regeneration therapy. Intensive randomized controlled clinical trials are required to confirm these findings and assess their clinical viability.