Abstract
This research examines the role of bio-activator, papaya latex in the synthesis of nanoporous carbon fibre made of the coal of coconut shell with aluminum oxide. The porous carbon was generated from coal activated by the papaya latex at the temperature of  for 4 h. The high energy milling (HEM) process up to 600 thousand cycles was applied to the porous carbon added with a precursor of aluminum oxide. The size and morphology of the particle are analyzed using SEM and TEM while the molecular group and the particle crystallization are analyzed with FTIR and XRD. The results show that papaya latex plays an effective role in breaking down the oxygen atoms in carbonyl group on the surface of carbon particle and in aluminum oxide. The oxygen breakdown creates pores in carbon particle and active site on the aluminum oxide. When the HEM is applied, the porous carbon particle breaks into fullerene-like nanoparticle whose yield is high and spontaneously connects the aluminum oxide as intercalation of particle whose structure turns into dimer
for 4 h. The high energy milling (HEM) process up to 600 thousand cycles was applied to the porous carbon added with a precursor of aluminum oxide. The size and morphology of the particle are analyzed using SEM and TEM while the molecular group and the particle crystallization are analyzed with FTIR and XRD. The results show that papaya latex plays an effective role in breaking down the oxygen atoms in carbonyl group on the surface of carbon particle and in aluminum oxide. The oxygen breakdown creates pores in carbon particle and active site on the aluminum oxide. When the HEM is applied, the porous carbon particle breaks into fullerene-like nanoparticle whose yield is high and spontaneously connects the aluminum oxide as intercalation of particle whose structure turns into dimer  (bucky-ball) in the form of carbon nanofibre.
(bucky-ball) in the form of carbon nanofibre.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanoparticle material is widely applied in many fields of study and technology, such as electronics, structural model, agriculture, and medicines. This is due to the fact that the contact surface of the nano-sized material is much wider so that its behavior is much better than in micro or macro size. The qualitative difference in the material behavior in nanoscale can be due to the effects of quantum mechanics which brings about its new physical and chemical characteristics for its very high surface to volume ratio [1].
Regarding the material of nanoparticle that has been proven for its better applicability, an attempt to synthesize the material is then widely developed using various precursors. To date, top-down approach (physical synthesis method) and bottom-up (chemical synthesis method) are two main approaches that are utilized to synthesize the nanoparticle material. In the top-down approach, the nanoparticle material is produced from the bulk size material by breaking it into nanoparticles using the energy or physical change. Meanwhile, in the bottom-up approach, the nanoparticle is synthesized through the chemical reactions between atoms and/or molecules [2]. The top-down approach generally requires auxiliary precursor/intercalation to fasten the process. By this, there are the mechanical process and chemical reaction that undergo. Hence it is also known as the mechanochemical reaction. Some auxiliary precursors that have been scrutinized are potassium salts such as  ,
,  , and metals, like Li, Na, K, Mg, Al, and Zn [3].
, and metals, like Li, Na, K, Mg, Al, and Zn [3].
Each method has its strength and weakness. The main strength of the physical synthesis method is the ability to shape the material into uniform nanoparticles. However, the main weakness of the method is the cost of investment for the tools that are high and the processing time that is relatively long. On the other hand, chemical synthesis method has its strength in terms of its control process, while its main weakness is the toxic characteristic of the chemical material used in a relatively high cost.
To reduce the negative impacts of the physical and chemical (identified for its high cost and toxicity) synthesis method, researchers have developed an alternative synthesis method using natural resources. The synthesis process is known as green synthesis, or also biosynthesis method. This method uses biological molecules that act as a reducer, including the microorganism, biomolecule, parts, and extract of the plant. Biosynthesis method follows a bottom-up approach and includes both reactions of oxidation and reduction. The reduction characteristic and antioxidant property of microbe enzymes [4, 5] or plant phytochemicals [6, 7] tend to reduce the metals into nanoparticles.
Some researchers reported that various plant extracts can be used as bio-activator to synthesize several kinds of metal into nanoparticle [8]. More specifically, there are some researchers that use some types of tree gums to synthesize the nanoparticles into various metals (Ag, Au, Pt, Pd, Fe, Cu, Se etc) and metal oxides ( , CuO, ZnO etc) [9]. Another study also serves the reduction process of silver nitride into nanosilver using the extract of papaya leaves [10]. The result shows that papain enzyme that exists in the extract of papaya leaves can reduce the solution of silver nitride into nanosilver ions.
, CuO, ZnO etc) [9]. Another study also serves the reduction process of silver nitride into nanosilver using the extract of papaya leaves [10]. The result shows that papain enzyme that exists in the extract of papaya leaves can reduce the solution of silver nitride into nanosilver ions.
Recently, a synthesis of carbon-based nanoparticle obtained from natural resources such as coconut shell, castor oil, tea waste and willow catkins plant has been done [11–14]. Many synthesis processes of nanoparticle whose material is natural carbon using active chemical element have been conducted [15]. Meanwhile, it is hardly found for a study that combines the use of natural carbon resources and natural active material or bio-activator. It is a gap that needs further study. Therefore, the present study investigates the role of an active natural material of papaya latex in producing carbon nanoporous and synthesizing it with aluminum oxides under mechanochemical reaction process to give carbon nanofibre. Porous carbon has played an important role as a host that is very well in absorbing, adsorption, separation, purification and catalytic reaction. Porous carbon is also important to accommodate and increase molecular interaction between host and foreign molecule [16]. The carbon nanofibre made from carbon nanoporous and aluminum oxide might play important role in nano power generator. The natural material used in this research is the charcoal of coconut shell. Papaya latex is used since it contains papain enzyme that has been proven for its ability to reduce the solution of silver nitride into nanosilver ions [10]. Whereas, aluminum oxide is used because it has been proved that aluminum could support the mechanochemical reaction [3].
2. Materials and methods
Precursor material used is charcoal powder made of the coconut shell with the main component in the weight: C about 91% and O about 8%. The powder is prepared using hardened steel crusher. The size of the powder is less than  (mesh 200). Papaya latex used is from the flower of male papaya with main compositions consisting of proteolytic enzymes, papain and chemopapain, glutamine cyclotransferase, chymopapain A, B and C, peptidase A and B and lysozymes [17]. As the auxiliary precursor, aluminum oxide particle is used.
(mesh 200). Papaya latex used is from the flower of male papaya with main compositions consisting of proteolytic enzymes, papain and chemopapain, glutamine cyclotransferase, chymopapain A, B and C, peptidase A and B and lysozymes [17]. As the auxiliary precursor, aluminum oxide particle is used.
Three combinations of precursor composition are as follow. The first one, only the charcoal powder of coconut shell (RCSC), second, the powder is added with papaya latex (E0) and, the last, the powder is added with papaya latex and aluminum oxide particle (E1). Two last combinations are put under the temperature of  for 4 h. Each combination is then given high mechanical energy from milling process (HEM) using custom-made shaker mill with hardened steel ball whose diameter is 6 mm. The number of the cycle for each combination is 600 thousand cycles. The prepared samples are analyzed by using scanning electron microscope (SEM) coupled with energy dispersive x-ray spectroscopy (EDX), FEI Inspect S-50; transmission electron microscope (TEM), JEOL JEM-1400; PANalytical X'pert PRO X-ray and Fourier transform infrared (FTIR) spectrometer, Shimadzu Prestige 21.
for 4 h. Each combination is then given high mechanical energy from milling process (HEM) using custom-made shaker mill with hardened steel ball whose diameter is 6 mm. The number of the cycle for each combination is 600 thousand cycles. The prepared samples are analyzed by using scanning electron microscope (SEM) coupled with energy dispersive x-ray spectroscopy (EDX), FEI Inspect S-50; transmission electron microscope (TEM), JEOL JEM-1400; PANalytical X'pert PRO X-ray and Fourier transform infrared (FTIR) spectrometer, Shimadzu Prestige 21.
3. Results and discussion
3.1. Microscopic analysis
Figure 1 shows the result of SEM and EDX test on nano-carbon material processed with HEM for 600 thousand cycles. Figure 1(a) is the basic material (RCSC) without any addition of papaya latex or aluminum oxide. Figure 1(b) is RCSC with the addition of papaya latex (E0). Figure 1(c) is RCSC with the addition of papaya latex and aluminum oxide (E1). Figures 1(d)–(f) are the result of EDX test on materials in figures 1(a)–(c), respectively.
Figure 1. The result of SEM and EDX test. SEM images of RCSC (a) without any addition of papaya latex or aluminum oxide, (b) with the addition of papaya latex (E0), and (c) with the addition of papaya latex and aluminum oxide (E1). (d)–(f): EDX spectra on materials in (a)–(c), respectively.
Download figure:
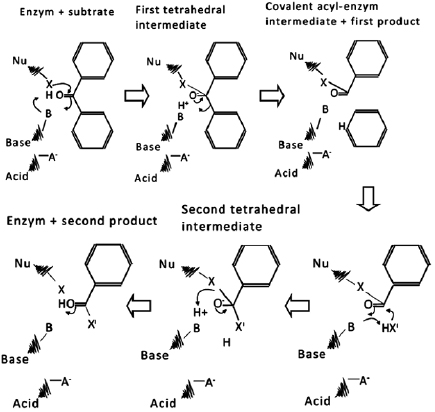
Standard image High-resolution imageFigures 1(a) and (b) show the significant difference in surface morphology and particle size. The particle size in figure 1(b) looks smaller and the size of the crack on its surface is bigger. The difference is due to the activity of the enzyme found in the papaya latex. The mechanism of enzyme activity is to break down the molecular group on the carbon surface so that cracks are created. The breakdown process is illustrated schematically in figure 2 considering that suggested by Shafee [18]. Enzyme creates the catalytic triad of acid-base-nucleophile. The triad then forms a relay network of material to polarize and activate the nucleophile. Carbonyl group on the carbon surface is then disrupted by the triad thus a bond of intermediate covalent is created. The bond of intermediate covalent is then hydrolyzed to regenerate the free enzyme. The mechanism keeps repeating throughout the condition of the environment, supporting the reaction to happen. As the molecular group is decomposed, then the carbon becomes more fragile so that the smaller size of the particle can be created even with the same level of milling energy. It means that the enzyme plays a significant role in increasing the yield during the mechanochemical process. The bigger the yield is, the more effective the synthesis process of nanoparticle it is. It also causes the energy consumption and processing time to be more efficient.
Figure 2. The process of decomposing the carbonyl group (C=O) by enzyme.
Download figure:
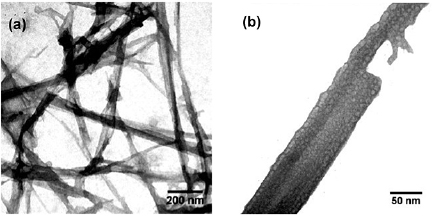
Standard image High-resolution imageThe presence of aluminum oxide in the milling process enables the formation of carbon fibre as seen in figure 1(c). Aluminum oxide functions as intercalation process that can form the series of dimmer buckyball  . The carbon fibre is morphologically solid as shown in figure 3. The formed structure resembles the nanocomposite of fullerene-like and aluminum oxide where each buckyball is connected with transformed aluminum oxide. The mechanical process of the fibre formation from the combination of the fullerene-like structure into the dimer
. The carbon fibre is morphologically solid as shown in figure 3. The formed structure resembles the nanocomposite of fullerene-like and aluminum oxide where each buckyball is connected with transformed aluminum oxide. The mechanical process of the fibre formation from the combination of the fullerene-like structure into the dimer  is shown in figure 4 considering that suggested by Komatsu et al [3]. The enzyme in the papaya latex plays a role in opening the bond of C=O on buckyball surface which then creates alkyl. The mechanical energy obtained from the milling process (HEM) results in highly activated local sites on the surface of buckyball that is ready to react [19, 20]. The active group influences the alkyl group to be released from the surface of buckyball and the O=Al bond on the precursor of aluminum oxide transforms and creates a bridge connecting two buckyballs. Thus respectively, the mechanochemical reaction happens from the milling process (HEM) connecting the dimmers so that then a fibre-like dimmer chain is created. Oxygen formed then binds all the carbon surface which then creates a group with a new function [21].
is shown in figure 4 considering that suggested by Komatsu et al [3]. The enzyme in the papaya latex plays a role in opening the bond of C=O on buckyball surface which then creates alkyl. The mechanical energy obtained from the milling process (HEM) results in highly activated local sites on the surface of buckyball that is ready to react [19, 20]. The active group influences the alkyl group to be released from the surface of buckyball and the O=Al bond on the precursor of aluminum oxide transforms and creates a bridge connecting two buckyballs. Thus respectively, the mechanochemical reaction happens from the milling process (HEM) connecting the dimmers so that then a fibre-like dimmer chain is created. Oxygen formed then binds all the carbon surface which then creates a group with a new function [21].
Figure 3. TEM images of sample E1 at different magnification.
Download figure:
Standard image High-resolution imageFigure 4. Proposed mechanism for the formation process of dimer  .
.
Download figure:
Standard image High-resolution imageThe result of EDX test shows that the addition of papaya latex does not change the composition significantly unless there is a decrease of the peak of intensity of O element and an increase of intensity of C element (figures 1(d) and (e)). It shows that there is a decrease of oxygen level as the result of the decomposition of carbonyl and carboxylate group. Meanwhile, an increase in the intensity of C element shows that enzyme mixed with charcoal has been denaturized into elements whose composing material are dominated by C element. The addition of aluminum oxide increases the intensity of the spectrum of O and Al elements as shown in figure 1(f). The increase of the number of O element can be explained as the result of the formation process of dimer  as shown in figure 4.
as shown in figure 4.
3.2. Spectroscopic analysis
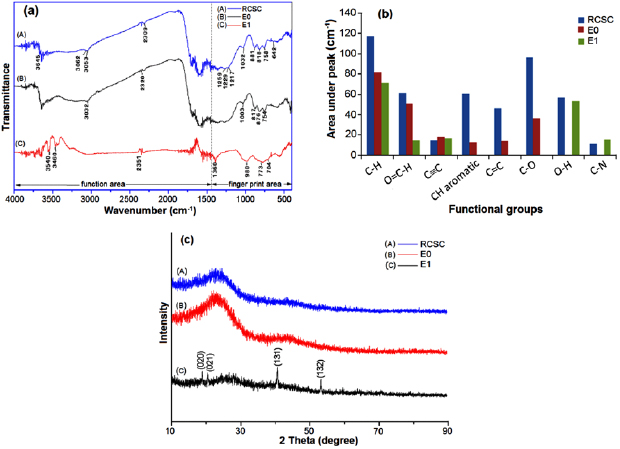
Figure 5 shows the result of FTIR and XRD test on samples RCSC, E0 and E1. Figure 5(a) shows FTIR spectra, figure 5(b) is the data area under the peak of the FTIR spectra, and figure 5(c) is XRD spectra. The shape of the curves (A) and (B) in figure 5(a) does not show a significant difference. The vast number of wave number values though their corresponding peaks do not appear clearly in curve (A) shows the presence of functional groups on the surface of the nanocarbon. The dominant reduction in the number of the wave number values in curve (B) while the main curve structure remains unchanged shows that the enzyme activity only decomposes the molecular functional group on carbon surface on the area of the functional group (wave number  ) or on the area of the fingerprint (wave number
) or on the area of the fingerprint (wave number  ) without changing the structure of nanocarbon.
) without changing the structure of nanocarbon.
Figure 5. (a) FTIR spectra, (b) data of area under the peak of (a), and (c) XRD spectra of samples RCSC, E0 and E1.
Download figure:
Standard image High-resolution imageThe groups reduced by enzyme are alkene and aromatic (C–H) at wave number  and
and  , carbonyl group (C=O) at wave number
, carbonyl group (C=O) at wave number  , alkyne group
, alkyne group  at wave number
at wave number  and carboxyl group (C–O) that are part of carbonyl group [22]. As the function group is decomposed on carbon surface, the pores are created and make the carbon fragile. It is verified by the size of the particle bullet that is smaller than the material added with papaya latex (figure 1(b)).
and carboxyl group (C–O) that are part of carbonyl group [22]. As the function group is decomposed on carbon surface, the pores are created and make the carbon fragile. It is verified by the size of the particle bullet that is smaller than the material added with papaya latex (figure 1(b)).
The addition of aluminum oxide in the mechanochemical reaction of carbon not only decomposes and eliminates several functional groups but also create new groups. The eliminated groups are carboxyl, aromatic and aromatic ring. The new emerging group is alcohol (O–H) and siano  . The presence of new groups is understandable since the condition of the environment in the mechanochemical reaction is airproof thus enables
. The presence of new groups is understandable since the condition of the environment in the mechanochemical reaction is airproof thus enables  and
and  molecules on air to give influence.
molecules on air to give influence.
An XRD test shows that the addition of papaya latex on the carbon does not change the structure. It can be seen from curve (A) and (B) in figure 5(c) where the early structure of carbon stays amorphous even after being added with papaya latex. It is also verified by the result of FTIR spectra where the curve does not undergo any change of shape. The change of structure happens on carbon processed with an addition of aluminum oxide precursor. The analysis of PANalytical X'pert High Score from the spectra obtains the crystal structure of monoclinic Bayerite with a chemical formula of  . Bayerite crystal in the series of dimer
. Bayerite crystal in the series of dimer  creates a structure of nano fullerene-like composite
creates a structure of nano fullerene-like composite  . Material with a similar structure of the composite of nano reduce-graphene oxide/
. Material with a similar structure of the composite of nano reduce-graphene oxide/ has a huge potential as a catalyst, fireproof, and semiconductor material [23, 24].
has a huge potential as a catalyst, fireproof, and semiconductor material [23, 24].
4. Conclusions
The enzyme in papaya latex is effective in decomposing carbonyl function group which then creates high porosity surface. This porous surface is easier to be broken into smaller particles (nano). It means enzyme plays a very important role in increasing the yield of nanoparticle in the process of mechanochemical synthesis. The addition of aluminum oxide in the mechanochemical reaction can combine the fullerene-like structure into a series of dimer  that resembles carbon fibre. The fibre has a structure of fullerene-like/bayerite nanocomposite that has a potential to be developed as fireproof and semiconductor material.
that resembles carbon fibre. The fibre has a structure of fullerene-like/bayerite nanocomposite that has a potential to be developed as fireproof and semiconductor material.
Acknowledgments
The author is grateful for the financial support from Directorate General of Higher Education, Ministry of Research, Technology and Higher Education of Indonesia with contract number 42/E/KPT/2017