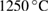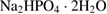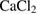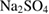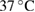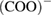Abstract
Each year million tons of fish bones and shellfish are caught, which were causing a serious environmental problem. Although these wastes contain valuable minerals, their use is not widespread. The main objective of the present study is the evaluation of the in vitro and in vivo behaviour of the extracted poly ( caprolactone)/nano-hydroxyapatite composites porous scaffolds. PCL/HA composites were prepared by an impregnation method, and the optimized composites were implanted in adult male albino (Sprague-Dawley strain) rats. The studied composites were evaluated as bone substitutes or fillers, and their degradation products were studied via biological and surgical examination. In vivo tests and biochemical studies were conducted to observe the changes found in blood. Biological studies showed that the decay of the PCL/HA scaffolds had no effect on the liver and kidney functions. They did not lead to carcinogenic or, oxidative effects and there was no oxygen radical's liberation that could damage the tissues. It also showed no inflammatory effect.
caprolactone)/nano-hydroxyapatite composites porous scaffolds. PCL/HA composites were prepared by an impregnation method, and the optimized composites were implanted in adult male albino (Sprague-Dawley strain) rats. The studied composites were evaluated as bone substitutes or fillers, and their degradation products were studied via biological and surgical examination. In vivo tests and biochemical studies were conducted to observe the changes found in blood. Biological studies showed that the decay of the PCL/HA scaffolds had no effect on the liver and kidney functions. They did not lead to carcinogenic or, oxidative effects and there was no oxygen radical's liberation that could damage the tissues. It also showed no inflammatory effect.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The interior portion of human bones is filled with spongy bone, which possesses a porous structure that provides space for blood vessels and marrow [1]. Bioceramics and bioglasses are calcium phosphate-based materials that are used as substitutes for bones. Porous bioceramic scaffolds are a valuable resource for the restoration of spongy bone, as bone cells can penetrate and disperse throughout and on the surface of their scaffolds [2, 3]. An appropriate open pore microstructure is necessary to permit both cell ingrowth and neovascularization from the surrounding tissue. This structure also ensures uniform cell distribution and in vivo cell migration, proliferation, and survival [4].
Highly porous hydroxyapatite (HA) scaffolds have been prepared from fish bone by Naga et al [5]. However, the brittleness and low tensile strength of the prepared HA scaffolds restricted their medical applications. The mechanical properties of HA scaffolds, especially their brittleness, can be improved by mixing HA with tough polymers [6]. Poly ( caprolactone) (PCL,
caprolactone) (PCL,  , a biodegradable polymer with high biocompatibility, is one of the main polymer groups used in biomaterials research. PCL is relatively hydrophobic and has a slow degradation rate, which makes it a good candidate for use as a bone substitute [7–9].
, a biodegradable polymer with high biocompatibility, is one of the main polymer groups used in biomaterials research. PCL is relatively hydrophobic and has a slow degradation rate, which makes it a good candidate for use as a bone substitute [7–9].
PCL/HA composites have been prepared by a non-alkoxide-based sol-gel method [10] and by the coating of porous HA scaffolds with PLC [11–13]. The first method led to the formation of a PCL/HA composite having well-distributed HA particles within a PCL matrix. The homogenous HA distribution improved the interaction between the ceramic and polymer phases and enhanced the bone cell recognition [10]. On the other hand, the second method maintained the high porosity and interconnectivity of the porous scaffold, which enhanced the mechanical properties. In addition, the presence of intergranular PCL particles resulted in the strengthening of the connection between particles, which prevented crack propagation or altered its pathway [11–13].
A solvent casting/particulate leaching method was used to fabricate PCL/fluoridated HA (PCL/FHA) nanocomposite scaffolds. An open pore structure with a pore size range of  was obtained by this method. The apatite phase was uniformly dispersed in the PCL polymeric matrix, and the produced scaffold had a network morphology. The compressive strength of the scaffold increased with an increase in the FHA ratio [14]. Many other methods, such as gas foaming/salt leaching, co-extrusion and gas foaming, had been used to prepare PCL/HA composites [15]. Selective laser sintering is another method that had been used for the preparation of PCL/HA composite scaffolds. The produced scaffolds were fully dense, with up to 30 vol % HA filler loading [16]. Additionally, unidirectional freezing of PCL/HA droplets dispersed in a hydrophilic poly (vinyl alcohol) (PVA) solution was used to fabricate a PCL/HA microsphere composite. The average size of the PCL/HA composite microspheres increased with increasing HA content along with a concomitant decrease in the pore size [17].
was obtained by this method. The apatite phase was uniformly dispersed in the PCL polymeric matrix, and the produced scaffold had a network morphology. The compressive strength of the scaffold increased with an increase in the FHA ratio [14]. Many other methods, such as gas foaming/salt leaching, co-extrusion and gas foaming, had been used to prepare PCL/HA composites [15]. Selective laser sintering is another method that had been used for the preparation of PCL/HA composite scaffolds. The produced scaffolds were fully dense, with up to 30 vol % HA filler loading [16]. Additionally, unidirectional freezing of PCL/HA droplets dispersed in a hydrophilic poly (vinyl alcohol) (PVA) solution was used to fabricate a PCL/HA microsphere composite. The average size of the PCL/HA composite microspheres increased with increasing HA content along with a concomitant decrease in the pore size [17].
Many authors have studied the in vitro characteristics of PCL/HA scaffolds [18–20]. An investigation of their in vitro degradation showed that PCL/n-HA scaffolds could retain relatively stable architectures and mechanical properties for at least six months [19]. On the other hand, biomimetic treatment of PCL/HA scaffolds provided the ability to form HA compounds in a simulated body fluid (SBF) solution [20].
Very few in vivo studies have been carried out on PCL/HA composites. Groppo et al [21] studied the effects of HA/PCL composites prepared by an impregnation method on the healing of subcritical calvarial bone defects in rats. No histological characteristics indicative of rejection of the implanted membrane were observed. The addition of HA to PCL enhanced the bone healing properties [22, 23]. Importantly, a higher in vitro expression of osteogenic differentiation markers was observed for PCL/HA composites than for PCL [21, 23].
PCL/HA composite scaffolds exhibited higher alkaline phosphatase (ALP) activity (ALP is an enzyme produced by differentiating osteoblasts and is responsible for constructing the bone matrix) and more extensive mineralization of the matrix than pure PCL scaffolds [18]. The PCL-coated HA scaffolds are nontoxic and possess an adequate 3D support for the attachment, proliferation and differentiation of MC3T3-E1 [24].
The present study aims to evaluate both the in vitro and in vivo behaviours of PCL/HA (derived from fish bones) composites. PCL/HA composites were prepared by the impregnation method, and the optimized composites were implanted in adult male albino (Sprague-Dawley strain) rats. The studied composites were evaluated as bone substitutes or fillers, and their degradation products were studied via biological and surgical examinations. This is the first report to examine the effects of biogenic PCL/HA composites on both the in vitro and in vivo behaviours.
2. Materials and methods
2.1. Materials
- Porous HA scaffolds prepared from biogenic HA powder and polymeric sponge template via heat treatment at
 for 3 h and PCL (Sigma-Aldrich Chemie Gmbh, Germany, P code 1001185525, lot # MKBH 7023 V, Molecular weight = 14,000 g/mole) were used for the preparation of PCL /HA composites.
for 3 h and PCL (Sigma-Aldrich Chemie Gmbh, Germany, P code 1001185525, lot # MKBH 7023 V, Molecular weight = 14,000 g/mole) were used for the preparation of PCL /HA composites. - SBF was prepared using chemically pure NaCl,
 , KCl,
, KCl,  ,
,  ,
,  , and
, and  (Sigma-Aldrich Chemie Gmbh, Germany).
(Sigma-Aldrich Chemie Gmbh, Germany). - In vivo studies were carried out on 40 adult male albino rats (Sprague-Dawley strain) of average weight (200–250 g). These animals were obtained from the laboratory animal house of the National Research Centre, Egypt. Blood samples were taken from these animals 2 and 6 months after the start of the experiment and kept for chemical analysis.
- Biochemical materials, biodiagnostic kits, intramuscular injection of 2% sodium thiopental, ketamine, betadine (povidone–iodine), extra pure (99.9%) absolute ethyl alcohol, 500 mg of Flumox antibiotic, 20 mg of Garmycin antibiotic, and 250 mg of Ketofan were used during and after the surgery.
2.2. Material characterization
The densification parameters, in terms of bulk density and apparent porosity, were measured by the water displacement method (ASTM C-20). The phases developed during firing were examined by x-ray diffraction (XRD) with monochromated  radiation (D 500, Siemens, Mannheim, Germany). SEM (Model XL 30, Philips, Eindhoven, Netherlands) was used to examine the microstructure of the studied samples. To assess the samples compressive strength, universal testing machine (LR 10 K plus 10 KN (2248 Ibf) type, Japan) with a crosshead displacement of 950 mm (37.4 in) was used. Extended versions were available with a displacement of up to 1435 mm (56.5 in), a speed range of 0.01–508 mm min−1, and a data sampling rate 8 kHz. The tested samples were in cubic form with a 1 cm length and 1 cm diameter.
radiation (D 500, Siemens, Mannheim, Germany). SEM (Model XL 30, Philips, Eindhoven, Netherlands) was used to examine the microstructure of the studied samples. To assess the samples compressive strength, universal testing machine (LR 10 K plus 10 KN (2248 Ibf) type, Japan) with a crosshead displacement of 950 mm (37.4 in) was used. Extended versions were available with a displacement of up to 1435 mm (56.5 in), a speed range of 0.01–508 mm min−1, and a data sampling rate 8 kHz. The tested samples were in cubic form with a 1 cm length and 1 cm diameter.
2.3. Methods
The surgical procedure was performed on the animals according to the Ethical Guidelines for Animal Care and the Ethics Committee of the National Research Centre of Egypt.
2.3.1. Preparation of HA scaffolds coated with PCL by a polymer impregnation method.
A polymer impregnation method was used to fabricate PCL-coated HA scaffolds [25, 26]. The abovementioned method coated all the interior and exterior surfaces of the HA scaffold with a PCL lining. Three different solutions of PCL with concentrations of 2, 4 and 6% w/v were prepared by dissolving the appropriate amount of PCL in 100 ml of dimethyl carbonate (DMC) under continuous stirring at  for 1 h, using a hotplate and a magnetic stirrer, and it was cooled after complete dissolution. Blocks of previously prepared HA scaffolds were immersed in the PCL solutions for different time intervals (5, 10, 15 min) and then dried in a fume hood for 12 h at room temperature.
for 1 h, using a hotplate and a magnetic stirrer, and it was cooled after complete dissolution. Blocks of previously prepared HA scaffolds were immersed in the PCL solutions for different time intervals (5, 10, 15 min) and then dried in a fume hood for 12 h at room temperature.
2.3.2. In vitro bioactivity test.
- Preparation of SBF.SBF was prepared using the technique described by Kokubo et al [27]
- In vitro bioactivity test.For the in vitro acellular tests, the studied samples were soaked for a fixed period (1 up to 4 weeks) in SBF solution, which possesses approximately the same ion composition of the human blood plasma. The temperature and the pH were preserved at
 and 7.4 respectively during the whole experiment. The obtained solutions were examined by inductively coupled plasma atomic emission spectroscopy (ICP-AES). At least 3 samples were used for each test. The samples surface was examined by both scanning electron microscopy (SEM) coupled with energy-dispersive x-ray analysis (EDS), and thin film XRD.
and 7.4 respectively during the whole experiment. The obtained solutions were examined by inductively coupled plasma atomic emission spectroscopy (ICP-AES). At least 3 samples were used for each test. The samples surface was examined by both scanning electron microscopy (SEM) coupled with energy-dispersive x-ray analysis (EDS), and thin film XRD.
2.3.3. In vivo test.
- Groups
- Control groupThis group contains 20 normal control rats divided into the following sub-groups:Sub-group 1: Contains 10 rats. Two months after the start of the experiment, blood samples were collected, and the rats were slaughtered.Sub-group 2: Contains 10 rats. Six months after the start of the experiment, blood samples were collected, and the rats were slaughtered.
- PCL/HA scaffold-treated groupThis group includes 20 normal albino rats treated with PCL/HA scaffolds divided into the following sub-groups:Sub-group 3: Contains 10 rats treated with PCL/HA scaffolds. Two months after the start of the experiment, blood samples were collected, and the rats were slaughtered.Sub-group 4: Contains 10 rats treated with PCL/HA scaffolds. Six months after the start of the experiment, blood samples were collected, and the rats were slaughtered.
- Surgical procedureFor the present study 40 mature male rats weight about 200 to 250 g were used. PCL/HA scaffolds were grafted in the introduced holes that executed in the rat's femur (20 rats were used). The 20 other rats were used as a control group, accordinglly they were left without any grafting.The rats were anesthetized with a dose of 40–45 mg kg−1 sodium thiopental given in the peritoneal cavity. The surgery area was shaved and sterilized. A hole of 2 mm diameter was produced in the sterilized rat's bone. Then, the entire hole was filled with a PCL/HA scaffold. An antibiotic (Flumox 500 mg) was used to avoid infections. The surgery site was then sewn.After grafting, 1 ml of Ketofan and 1 ml of Garmycin were administered into the intraperitoneal cavities of the rats for 3 d after the operation. Ten animals were slaughtered for evaluation 2 months after the grafting materials were implanted, and another ten were slaughtered 6 months after implantation.
- Post-surgery assessment
- Clinical examinationAfter the operation and during the whole experimental duration every rat was observed carefully for the expected complications.
- SEM/EDS examinationsThe microstructure and Ca/P ration were studied on polished bone's cross sections using SEM coupled with EDS. The Ca/P ratio of the anew created bones was compared with those obtained from the control group.
- Histological studyHaematoxylin and eosin (H&E) was used to stain the studied bones thin sections. The microscopic examination of the thin sections was carried out with a Leica optical polarization microscope.
- The biological evaluation for the safety of the grafted scaffoldsThe study of the biological effect of the grafted scaffolds was necessary to evaluate the influence of scaffolds' decay products on the rat's organs functions. Accordingly, the following analyses were performed: (1) Liver and kidney functions tests. (2) Measurement of tumour markers. (3) Determination of free radical biomarkers known as lipid peroxides (Malondialdehyde). (4) Determination of nitric oxide production in serum as an indicator of the inflammatory effect of the implanted materials. (5) Statistical analysis.
3. Results and discussion
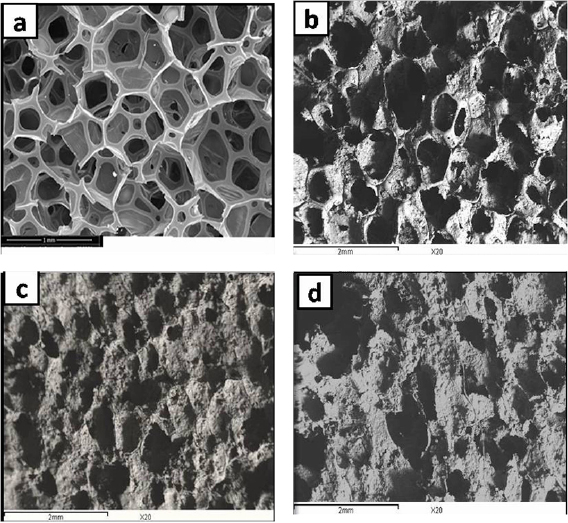
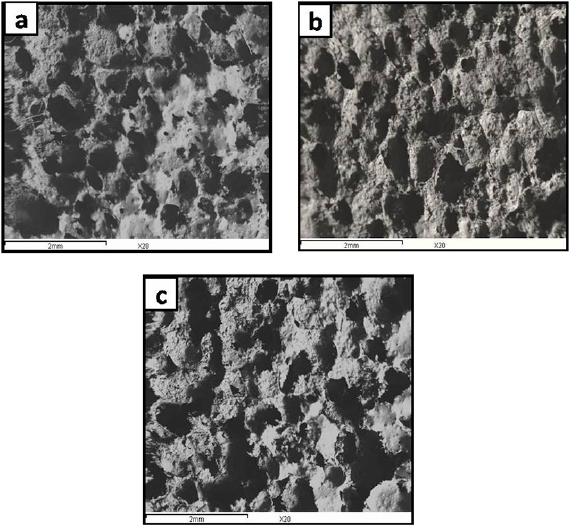
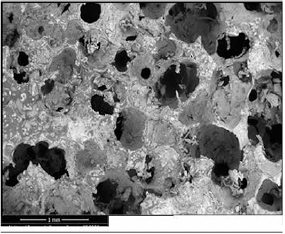
Due to the importance of both the scaffold porosity and pore connection, the morphological structure and compressive strength of the HA scaffolds coated with PCL concentrations of 2, 4 and 6% w/v for time intervals of 5, 10 and 15 min were investigated. The appropriate PCL concentration and soaking time were determined on the basis of the obtained results. Figure 1(a) presents the typical morphology of an untreated sponge, while the SEM images of the HA scaffolds coated with 2, 4 and 6% w/v PCL for 10 min were presented in figures 1(b)–(d), respectively. The 2% w/v PCL solution was too dilute and could not coat the scaffold properly. In contrast, the 6% w/v PCL solution was too viscous and could not fully diffuse through the pores. Thus, the 4% w/v PCL solution has proved to be the most appropriate. To determine a suitable soaking time, the HA scaffolds were immersed in the 4% w/v PCL solution for 5, 10 and 15 min. The results indicated that 10 min was the most suitable soaking time, figures 2(a)–(c). Accordingly, an impregnation time of 10 min was utilized to prepare the 4% w/v PCL/HA porous scaffolds. The coated HA scaffolds possessed round interconnected macropores and maintained the initial sponge structure (figure 3).
Figure 1. (a) SEM micrograph for untreated sponge, (b)–(d) SEM of HA scaffolds coated with 2, 4 and 6% PCL for 10 min.
Download figure:
Standard image High-resolution imageFigure 2. (a)–(c) SEM micrograph for HA scaffolds coated with 4% PCL for 5, 10 & 15 min.
Download figure:
Standard image High-resolution imageFigure 3. The SEM micrograph for HA coated with 4% w/v PCL for 10 min.
Download figure:
Standard image High-resolution image3.1. Characterization of the PCL/HA composites
3.1.1. Physical properties of the PCL/HA scaffolds coated for 10 min.
The results showed that coating the HA scaffolds increased the bulk density and decreased the apparent porosity (table 1). The bulk density increased by 1.75 times, while the apparent porosity decreased by 1.25 times. This improvement in the physical properties is due to the PCL coating, which penetrated the pores and small cracks, resulting in a porosity reduction and a density enhancement.
Table 1. Physical and mechanical properties of blank HA and PCL/HA composites.
| Scaffold type | Bulk density,  |
Apparent porosity, % | Compressive strength, MPa | Bending strength, MPa |
|---|---|---|---|---|
| blank HA (5) |  |
 |
0.13 ± 0.01 |  |
| 4% w/v PCL/HA | 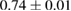 |
 |
 |
 |
3.1.2. Mechanical properties.
Table 1 illustrated the results of the compressive and bending strengths of the HA scaffolds coated with PCL. The results indicated that the PCL coating had a positive effect on the compressive strength, which increased from  MPa for the untreated HA scaffold to
MPa for the untreated HA scaffold to  MPa for the HA scaffolds coated with PCL for 10 min. The improvement in the compressive strength of the coated scaffolds is believed to be due to the PCL polymer, which imparts flexibility in the brittle HA scaffold [11]. The PCL coating plays the same role as the organic component on raw bones, which improves the compressive strength of raw bone by restraining crack propagation through the redistribution of the compressive stress and dispersion of the energy during deformation [24].
MPa for the HA scaffolds coated with PCL for 10 min. The improvement in the compressive strength of the coated scaffolds is believed to be due to the PCL polymer, which imparts flexibility in the brittle HA scaffold [11]. The PCL coating plays the same role as the organic component on raw bones, which improves the compressive strength of raw bone by restraining crack propagation through the redistribution of the compressive stress and dispersion of the energy during deformation [24].
The bending strength of the PCL/HA composites increased from  for untreated HA to
for untreated HA to  MPa for the PCL/HA composites. This improvement in the bending strength of the composites was due to the enhancement of the physical properties. The use of HA/PCL composites gave the chance of tailoring the mechanical properties of the final product. It is well known that polymers are flexible, while HA is brittle and display poor fracture resistance. The integration of PCL and HA leads to the formation of a new material; composite; with amended stiffness and compression strength. Such composites presented an additional flexibility degree convenient for the mechanical and physiological demands of the biomaterials.
MPa for the PCL/HA composites. This improvement in the bending strength of the composites was due to the enhancement of the physical properties. The use of HA/PCL composites gave the chance of tailoring the mechanical properties of the final product. It is well known that polymers are flexible, while HA is brittle and display poor fracture resistance. The integration of PCL and HA leads to the formation of a new material; composite; with amended stiffness and compression strength. Such composites presented an additional flexibility degree convenient for the mechanical and physiological demands of the biomaterials.
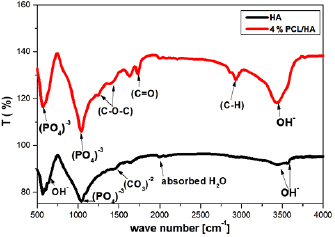
Figure 4 shows the Fourier transform infrared (FT-IR) spectrum of the PCL/HA composite. The figure showed that the bands consistent with PCL are as follows: (1) bands at 2864 and  , which are related to
, which are related to  ; (2)
; (2)  stretching vibration mode at
stretching vibration mode at  , which indicates the presence of an aliphatic ester; (3)
, which indicates the presence of an aliphatic ester; (3)  stretching band at
stretching band at  ; and (4) methane stretching vibration
; and (4) methane stretching vibration  at
at  . The band at
. The band at  is assigned to a protonated carboxylate group. The presence of such a group is indicative of a reaction between the carboxylate groups and
is assigned to a protonated carboxylate group. The presence of such a group is indicative of a reaction between the carboxylate groups and  ions [28]. The following characteristic bands of
ions [28]. The following characteristic bands of  are observed: (1) asymmetric stretching vibration mode (
are observed: (1) asymmetric stretching vibration mode ( ) at
) at  and (2) OPO vibration mode (
and (2) OPO vibration mode ( ) at 567
) at 567  . The stretching mode of the
. The stretching mode of the  groups is observed at
groups is observed at  [24].
[24].
Figure 4. FTIR spectrum for HA and 4% w/v PCL/HA composites.
Download figure:
Standard image High-resolution imageOn the other hand, it was easy to recognize the characteristic bands of HA, which were:  (1040, 600 and
(1040, 600 and  ,
,  , and
, and  ).
).  group band was found as a small absorption band at
group band was found as a small absorption band at  . It was associated with the stretching mode of hydroxyl group. The band at
. It was associated with the stretching mode of hydroxyl group. The band at  was also assigned for the
was also assigned for the  group [29]. The vibrational frequencies for
group [29]. The vibrational frequencies for  can be detected around 1400 and at
can be detected around 1400 and at  for
for  [30]. We believe that the carbonate group existed as the polymeric template residue.
[30]. We believe that the carbonate group existed as the polymeric template residue.
3.2. Bioactivity measurements and in vitro tests
3.2.1. ICP tests.
The concentrations of  and
and  ions in the SBF solution after immersion of the PCL/HA scaffolds for different time intervals are shown in figure 5. The
ions in the SBF solution after immersion of the PCL/HA scaffolds for different time intervals are shown in figure 5. The  and
and  ion concentrations decreased sharply over the first 2 weeks, and the decrease in the ion concentrations began to slow during the third week and then remained approximately stable during the fourth week.
ion concentrations decreased sharply over the first 2 weeks, and the decrease in the ion concentrations began to slow during the third week and then remained approximately stable during the fourth week.  and
and  ions are considered apatite nucleation agents, as they facilitate the formation and deposition of apatite crystals on the surface of the PCL/HA scaffolds. The formed apatite layer grows spontaneously by consuming the
ions are considered apatite nucleation agents, as they facilitate the formation and deposition of apatite crystals on the surface of the PCL/HA scaffolds. The formed apatite layer grows spontaneously by consuming the  and
and  ions in the SBF. The highly negative carboxylate groups in PCL that formed from the release of an acidic degradation product attracted
ions in the SBF. The highly negative carboxylate groups in PCL that formed from the release of an acidic degradation product attracted  ions to form complexes that acted as nucleation sites for apatite crystal formation [28]. The negative charges of the PCL hydroxyl groups and phosphate ions attract the positively charged
ions to form complexes that acted as nucleation sites for apatite crystal formation [28]. The negative charges of the PCL hydroxyl groups and phosphate ions attract the positively charged  ions to form a complex rich in
ions to form a complex rich in  . The formed complex reacts with the negative
. The formed complex reacts with the negative  ions to produce HA crystals [31].
ions to produce HA crystals [31].
Figure 5.  and
and  ions of concentrations versus immersion time periods for 4% w/v PCL/HA scaffolds.
ions of concentrations versus immersion time periods for 4% w/v PCL/HA scaffolds.
Download figure:
Standard image High-resolution image3.2.2. Thin film XRD analysis.
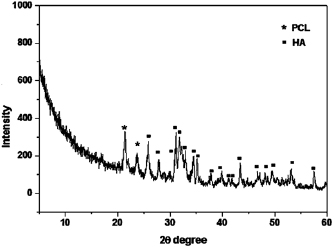
The thin film x-ray pattern of PCL/HA (figure 6) showed that the formed layer is composed mainly of pure HA as the dominant phase and PCL as the minor phase. PCL diffraction peaks are observed at  and
and  , while the HA peaks are consistent with the JCPDS (76-0694) standard data.
, while the HA peaks are consistent with the JCPDS (76-0694) standard data.
Figure 6. Thin film x-ray for 4% w/v PCL/HA scaffolds after immersion in SBF for 4 weeks.
Download figure:
Standard image High-resolution image3.2.3. Microstructural characterisation by SEM and EDS.
Figures 7(a) and (b) shows the SEM micrographs of the PCL/HA scaffold surface before (a) and after (b) immersion in SBF for 4 weeks. Figure 7(b) showed that the surface of the PCL/HA scaffold was completely covered with fine and coarse hemispherical HA crystals. HA nucleation may occur either homogeneously or heterogeneously depending on the nucleation mechanism [32]. In the present study, because the proper sites for nucleation were available and the interfacial energy of the crystalline HA was insufficient for homogeneous nucleation, HA nucleated and grew heterogeneously as hemispherical crystals [33]. EDS analysis (figure 8) was performed on the HA layer on the surface of the PCL/HA scaffold after immersion in the SBF solution for 4 weeks. The Ca/P ratio was 1.56, which is very near to the Ca/P value of living bone apatite. It was reported that biological apatite has a Ca/P ratio between 1.55 and 1.85. The Ca/P ratio depends on the bone function and type. The results of the in vitro study confirmed the excellent bioactivity for the PCL/HA scaffolds [16, 34].
Figure 7. SEM micrograph for 4% w/v PCL/HA scaffold before (a) and after (b) immersion in SBF for 4 weeks and energy-dispersive x-ray analysis (EDX) of the formed HA.
Download figure:
Standard image High-resolution imageFigure 8. (a)–(c) Scanning electron microscope micrographs for different magnifications of cross-section of bone defect created in femur of albino rat and grafted with 4% w/v PCL/HA scaffold for two months. (d) Illustrates the EDX analysis of newly formed bone.
Download figure:
Standard image High-resolution image3.2.4. Degradation study.
The biodegradability is one of the most important standards for ideal biomedical scaffolds. PCL is a water-soluble polymer consisting of an aliphatic polyester with a high molecular weight [35] that possess a hydrolysable ester bond, which enhances its degradability. To determine the scaffold biodegradability, the water uptake capability (swelling) and weight loss of the studied scaffolds were examined. The weight loss of the PCL/HA scaffolds steadily increased with increasing immersion time until it reached  after 28 d of immersion in doubly distilled water (table 2). In comparison to pure HA scaffolds, the PCL/HA scaffolds showed a faster degradation rate (blank HA:
after 28 d of immersion in doubly distilled water (table 2). In comparison to pure HA scaffolds, the PCL/HA scaffolds showed a faster degradation rate (blank HA:  , PCL/HA:
, PCL/HA:  ). The change in the PCL/HA degradation rate was due to the presence of PCL, which possessed a higher degradation rate than blank HA.
). The change in the PCL/HA degradation rate was due to the presence of PCL, which possessed a higher degradation rate than blank HA.
Table 2. The weight loss (%) of 4% PCL/HA scaffolds after immersion in double distilled water for 28 d.
| Time, days | Weight loss, (%) | |
|---|---|---|
| 4% PCL/HA | HA | |
| 0.125 | 0.027 ± 0.002 | 0.081 ± 0.003 |
| 0.25 | 0.044 ± 0.003 | 0.174 ± 0.099 |
| 0.5 | 0.067 ± 0.003 | 0.354 ± 0.004 |
| 1 | 0.304 ± 0.004 | 0. 593 ± 0.034 |
| 3 | 1.12 ± 0.010 | 1.11 ± 0.015 |
| 7 | 2.40 ± 0.030 | 1.76 ± 0.120 |
| 14 | 4.26 ± 0.070 | 2.64 ± 0.035 |
| 21 | 6.94 ± 0.080 | 3.85 ± 0.054 |
| 28 | 10.78 ± 0.070 | 5.51 ± 0.050 |
3.3. In vivo bioactivity assessment of the PCL/HA scaffolds
3.3.1. Biological studies for evaluating the safety of the implanted PCL/HA composites.
- Liver function testsTables 3 and 4 listed the results of the liver function activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and ALP for the control group rats and rats grafted with PCL/HA scaffold for 2 and 6 months. The results showed that the ALT and AST activities in the animals grafted for 2 months were lower than those in the control group rats. On the other hand, the ALP activity in the animals grafted for 2 months was higher than those for the control group. Table 4 showed that the animals grafted for 6 months had lower AST and ALP activities and a higher ALT activity than the control group. In all cases, the activity differences between the grafted animals and the control group were not considerable (
 ). The decrease in the ALT activity of the grafted animals relative to that of the control group is believed to be due to the ageing of the rats [36, 37] and the decomposition of the grafted material. The ALT/AST ratio was less than 1, which indicates the absence of inflammation or liver damage. The abovementioned results are in good agreement with the study carried out by Injac et al [37]. The obtained liver function results indicate that the grafted animals possessed normal liver functions and that the degradation products of the PCL/HA scaffold did not cause defects of the liver.
). The decrease in the ALT activity of the grafted animals relative to that of the control group is believed to be due to the ageing of the rats [36, 37] and the decomposition of the grafted material. The ALT/AST ratio was less than 1, which indicates the absence of inflammation or liver damage. The abovementioned results are in good agreement with the study carried out by Injac et al [37]. The obtained liver function results indicate that the grafted animals possessed normal liver functions and that the degradation products of the PCL/HA scaffold did not cause defects of the liver. - Kidney function testsThe results of the kidney function tests, determined by the serum creatinine and urea levels, for the animals grafted with the PCL/HA scaffold for 2 and 6 months in comparison to the results for the control group are given in tables 3 and 4. The results show an increase in both the serum creatinine and urea levels over those of the control group. The differences statistically inconsiderable
 , where
, where  indicates statistical significance by the SPSS program. The details of the formula used were given by Dunnett [38]. The present results possessed only slight differences between the short-term (2 months) and long-term (6 months) grafted groups, which indicates that the kidney function was normal. In other words, the degradation products of the PCL/HA scaffold did not cause kidney failure.
indicates statistical significance by the SPSS program. The details of the formula used were given by Dunnett [38]. The present results possessed only slight differences between the short-term (2 months) and long-term (6 months) grafted groups, which indicates that the kidney function was normal. In other words, the degradation products of the PCL/HA scaffold did not cause kidney failure. - Measurement of tumour markersThe
 fucosidase (AFU) and arginase activities in the serum of both the control group and the PCL/HA grafted groups are given in tables 3 and 4. The results demonstrated that implantation of the PCL/HA scaffold for 2 and 6 months decreased the tumour markers relative to those of the control group. The difference was inconsiderable
fucosidase (AFU) and arginase activities in the serum of both the control group and the PCL/HA grafted groups are given in tables 3 and 4. The results demonstrated that implantation of the PCL/HA scaffold for 2 and 6 months decreased the tumour markers relative to those of the control group. The difference was inconsiderable  . The abovementioned results indicate that the grafted PCL/HA scaffold had no carcinogenic effects.
. The abovementioned results indicate that the grafted PCL/HA scaffold had no carcinogenic effects. - Determination of the free radical biomarker lipid peroxide (malondialdehyde)The results shown in tables 3 and 4 indicate that the grafted animals had higher levels of serum lipid peroxide than the control group. The serum lipid peroxide test provides a measure of in vivo cellular injury in animals. Although the lipid peroxide activity of the grafted animals was higher than that of the control group, the statistical analysis showed no significant difference
 .
. - Determination of nitric oxide production in serumThe level of nitric oxide in serum is an indicator of the inflammatory effect of the grafted materials. The results show that the grafted animals displayed a high nitric oxide activity after 2 and 6 months. However, the statistical difference between the grafted animals and the control group was not significant
 . Therefore, grafting with the PCL/HA scaffold did not cause any inflammatory effects.
. Therefore, grafting with the PCL/HA scaffold did not cause any inflammatory effects.
Table 3. The liver and kidney functions, tumor markers, nitric oxide and lipid peroxide levels measured in serum of animals grafted with PCL/HA scaffolds in comparison with control group; 2 months implantation.
| Sample code | AST (U ml−1) | ALT (U ml−1) | ALP (IU 1−1) | Creatinine (mg dl−1) | Urea (g dl−1) | AFU (U l−1) | Arginase (U l−1) | Lipid peroxide (nmol ml−1) | Nitric oxide (nmole l−1) |
|---|---|---|---|---|---|---|---|---|---|
| Control group | 258 ± 8.70 | 49 ± 2.35 | 395 ± 4.16 | 0.37 ± 0.02 | 43 ± 1.28 | 4.7 ± 0.33 | 117 ± 2.7 | 1.38 ± 0.19 | 1.84 ± 0.26 |
| PCL/HA group | 155 ± 8.3 | 44 ± 2.86 | 538 ± 5.85 | 0.62 ± 0.03 | 46 ± 1.47 | 3.86 ± 0.53 | 115 ± 3.61 | 3.26 ± 0.32 | 2.07 ± 0.16 |
Table 4. The liver and kidney functions, tumour markers, nitric oxide and lipid peroxide levels measured in serum of animals grafted with PCL/HA scaffolds in comparison with control group; 6 months implantation.
| Sample code | AST (U ml−1) | ALT (U ml−1) | ALP (IU 1−1) | Creatinine (mg dl−1) | Urea (g dl−1) | AFU (U l−1) | Arginase (U l−1) | Lipid peroxide (nmol ml−1) | Nitric oxide (nmole l−1) |
|---|---|---|---|---|---|---|---|---|---|
| Control group | 176 ± 5.9 | 42 ± 2.04 | 486 ± 6.58 | 0.76 ± 0.06 | 43 ± 1.41 | 2.9 ± 0.15 | 156 ± 2.63 | 2.64 ± 0.58 | 1.96 ± 0.41 |
| PCL/HA group | 139 ± 7.9 | 45 ± 3.89 | 346 ± 5.48 | 0.79 ± 0.05 | 49 ± 3.25 | 2.1 ± 0.39 | 182 ± 2.21 | 5.82 ± 0.39 | 2.32 ± 0.31 |
3.3.2. SEM and EDS analysis.
- Two months after the surgical operationFigures 8(a)–(c) shows different magnifications of SEM micrographs of the bone defect cross-section in albino rat femur 2 months after grafting with the PCL/HA scaffold. The images revealed that the defect is nearly filled with new bone; however, the defect is still not completely bridged in the middle. Some collagen fibres were observed inside the unbridged portion of the defect. Additionally, remnants of the scaffold were found in the defect. Some of these remnants were located inside the defect, while the majority extended from the defect throughout the BM cavity. Note that most of the scaffold remnants inside the BM cavity were completely surrounded by the newly formed bone, which indicates that the scaffolds were able to induce bone regeneration throughout the bone defect as well as inside the BM cavity. The abovementioned results reveal that the scaffold is highly bioactive. The EDS analysis results of the newly formed bone in the defect are shown in figure 8(d). The Ca/P molar ratio of the newly formed bone was 1.59, which was less than that of the control group (1.79), indicating that the newly formed bone was still not well mineralized.
- Six months after the surgical operationFigures 9(a)–(c) displays different magnifications of the SEM micrographs of the bone defect cross-section in an albino rat femur 6 months after grafting with the PCL/HA scaffold. A closer look at figure 9 reveals that the bone defect was completely filled with newly formed bone and that the new bone covered the middle of the defect. Minor fragments of the PCL/HA scaffold were still present in the bone defect, and the BM cavity was still filled with portions of the PCL/HA scaffold, which were surrounded by the newly formed bone. Figure 9(c) shows one of those portions enclosed in a blue circle. The EDS analysis results of the newly formed bone that filled the defect are shown in figure 9(d). The analysis indicates that the bone formed at this advanced stage (six months) became more mineralized than that after two months, as the Ca/P molar ratio increased from 1.59 to approximately 1.66. This ratio is closer to that of the normal bone obtained from the control group (1.79). The in vivo trials proved that the PCL/HA scaffold efficiently restored well mineralized bone in the bone defect, and this newly formed bone was comparable to the normal bone of the control group after only six months. The bioactivity of PCL/HA scaffold could be attributed to the following reasons:The scaffold contains PCL, which possesses ester bonds
 . Those negatively charged groups could attract
. Those negatively charged groups could attract  ions from the body fluids, and these ions could in turn attract
ions from the body fluids, and these ions could in turn attract  ions from the body fluids to form an apatite layer on the surface of the scaffold. This layer is considered very important for the integration of any material within normal bone in the body.The scaffold consists of HA, which releases
ions from the body fluids to form an apatite layer on the surface of the scaffold. This layer is considered very important for the integration of any material within normal bone in the body.The scaffold consists of HA, which releases  and
and  ions into solution. The release of these ions accelerated apatite precipitation on the scaffold surface and improved its integration in normal bone.The scaffold is highly porous. This porous structure facilitated the invasion, penetration and spreading of cells throughout the scaffold and thus accelerated bone regeneration and reduction of the bone defect.
ions into solution. The release of these ions accelerated apatite precipitation on the scaffold surface and improved its integration in normal bone.The scaffold is highly porous. This porous structure facilitated the invasion, penetration and spreading of cells throughout the scaffold and thus accelerated bone regeneration and reduction of the bone defect.
Figure 9. (a)–(c) Shows scanning electron microscope micrographs that demonstrate different magnifications of cross-section of bone defect made in femur of albino rat and grafted with 4% w/v PCL/HA scaffold for six months. (d) Illustrates the EDX analysis of newly formed bone.
Download figure:
Standard image High-resolution image3.3.3. Histological analysis.
- Two months after the surgical operationFigure 10(a) shows a histological section of a femur bone defect grafted with PCL/HA two months after the surgical procedure. The figure revealed that the newly developed bone (nb) grew from the edges of the defect towards the centre and that the middle section of the defect was not completely restored. Moreover, blood vessels (bv) developed throughout the nb, which indicated that the nb had a good supply of nutrients and oxygen. Several active osteoblasts (ob) and secreting collagen fibres (cf) were observed across the non-bridged portion of the defect. Those fibres joined together at numerous positions to form osteoid tissue. Pieces of the scaffold (indicated by the pink and blue squares) were observed near the inner portion of the defect. These pieces faced the BM, extended throughout the BM cavity, and were surrounded by the newly generated bone. Osteoblasts spread over those pieces and led to the production of new bone, which indicated that the grafted scaffold was not toxic to osteoblasts. Examining the newly developed bone inside the defect as well as in the BM cavity indicated that the formed bone tissue was woven bone and was still immature, as several osteocytes (oc) were embedded in this bone. Figure 10(b) showed a higher magnification image of the area within the pink square. In the figure, a piece of the scaffold surrounded by new bone spread from the bone defect into the BM cavity. Several osteoblasts were observed on the new bone surface, indicating that the scaffold was biocompatible with those cells. On the other hand, figures 10(c) and (d) show a higher magnification image of another piece of the scaffold (enclosed within the blue square) and illustrated that the new bone spread through the scaffold. This result proved that bone cells were able to attach to the scaffold, disseminated through its porous structure, proliferated and interacted with the components of the scaffold, finally leading to bone formation inside the porous structure. Moreover, a newly developed blood vessel filled with red blood cells and surrounded by osteoblasts (as indicated by the yellow dotted circle) passed through the scaffold. This blood vessel could deliver nutrients and oxygen to the cells and remove their waste products.
- Six months after the surgical operationFigure 11(a) demonstrates a histological section of a femur bone defect six months after the surgical procedure. The defect was filled with well mature lamellar bone, and numerous Haversian systems were distributed throughout the lamellar bone, as shown by the dotted blue circles. Most of the scaffold fragments were degraded at this stage, and very few were observed in the bone defect. However, some fragments still existed in the BM cavity. Figure 11(b) illustrates a magnified view of the area enclosed within the yellow square in figure 11(a). This image showed well developed Haversian systems denoted by blue circles. The histological analysis revealed that the PCL/HA scaffold was bioactive and biocompatible and had a strong ability to restore normal and well mature lamellar bone in a bone defect. The PCL/HA composite can be concluded to induce the generation of well mature lamellar bone throughout the entire defect. Furthermore, the remodelling process began in the bone tissue surrounding the PCL/HA scaffold very soon after implantation in the defect.
Figure 10. (a)–(d) Shows different magnifications of a histological section of femur bone defect grafted with 4% w/v PCL/HA two month post the surgical procedure.
Download figure:
Standard image High-resolution imageFigure 11. (a) and (b) Shows different magnifications of a histological section of femur bone defect grafted with 4% w/v PCL/HA scaffold six months post-surgical operation.
Download figure:
Standard image High-resolution image4. Conclusions
The reinforcement of HA scaffolds by 4% w/v PCL for 10 min enhanced the mechanical properties of the scaffolds. In vitro tests indicated that the PCL/HA scaffolds had excellent bioactivity. The improvement in the bioactivity of the scaffolds was due to the presence of carboxylate  groups in the composites that could attract
groups in the composites that could attract  ions from the SBF solution and act as nucleation sites. The studied rats were remained alive after the surgery. They did not suffer from any complications, which means that the grafted PCL/HA scaffold did not cause any histopathology or biocompatibility issues with the circumferential osseous tissue. Biological studies for evaluating the safety of the implanted PCL/HA scaffolds showed that the grafted animals had normal liver and kidney functions, which indicates that the degradation products of the implanted materials did not lead to liver or kidney disorders. The results also indicated that PCL/HA scaffold had no carcinogenic or inflammatory effects. Two months post-surgery, the Ca/P molar ratio of the newly formed bone surrounding the scaffold was less than that of the control group animals, which indicates that the newly formed bone was not well mineralized. Six months post-surgery, the bone defect was completely healed. The Ca/P molar ratio was identical to the untreated group, meaning that the new bone was well mineralized. Finally, we recommend PCL/HA scaffolds to be used as bone graft.
ions from the SBF solution and act as nucleation sites. The studied rats were remained alive after the surgery. They did not suffer from any complications, which means that the grafted PCL/HA scaffold did not cause any histopathology or biocompatibility issues with the circumferential osseous tissue. Biological studies for evaluating the safety of the implanted PCL/HA scaffolds showed that the grafted animals had normal liver and kidney functions, which indicates that the degradation products of the implanted materials did not lead to liver or kidney disorders. The results also indicated that PCL/HA scaffold had no carcinogenic or inflammatory effects. Two months post-surgery, the Ca/P molar ratio of the newly formed bone surrounding the scaffold was less than that of the control group animals, which indicates that the newly formed bone was not well mineralized. Six months post-surgery, the bone defect was completely healed. The Ca/P molar ratio was identical to the untreated group, meaning that the new bone was well mineralized. Finally, we recommend PCL/HA scaffolds to be used as bone graft.
Acknowledgment
The authors admit that there are no competing interests, the study does not involve human subject and that, Science & Technology Development Fund (STDF), Egypt has financed their study, German-Egyptian Research Fund (GERF) Projects programme, Project ID 23036.