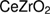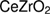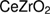Abstract
Semi-crystalline and crystalline ceria-zirconia,  nanoparticles had been successfully synthesized by water/oil microemulsion method at room temperature without and with calcination process. The basic understanding on the influence of microemulsion system and the relation of calcination process on the microstructure of nanoparticles was studied. Without calcination, the semi-crystalline nanoparticles with the size in the range of
nanoparticles had been successfully synthesized by water/oil microemulsion method at room temperature without and with calcination process. The basic understanding on the influence of microemulsion system and the relation of calcination process on the microstructure of nanoparticles was studied. Without calcination, the semi-crystalline nanoparticles with the size in the range of  were produced. This size was consistent to the droplet size of microemulsion and the XRD crystallite size. In
were produced. This size was consistent to the droplet size of microemulsion and the XRD crystallite size. In  , zirconia ions incorporated into the ceria structure to form a complete solid solution of nanoparticles. The specific surface area was
, zirconia ions incorporated into the ceria structure to form a complete solid solution of nanoparticles. The specific surface area was  and it was found as a highly microporous material. While, after calcination process, the degree of crystallinity of nanoparticles increased and the fully crystalline nanoparticles were produced. The crystals and particles size grew up to 12 nm, while the surface area reduced to
and it was found as a highly microporous material. While, after calcination process, the degree of crystallinity of nanoparticles increased and the fully crystalline nanoparticles were produced. The crystals and particles size grew up to 12 nm, while the surface area reduced to  . However, both nanoparticles, with and without calcination, were consistent as a cubic structure. By comparing the properties of both nanoparticles samples, it can be concluded that the nanoparticles
. However, both nanoparticles, with and without calcination, were consistent as a cubic structure. By comparing the properties of both nanoparticles samples, it can be concluded that the nanoparticles  are able to be synthesized using the microemulsion method at room temperature without calcination process and it is suitable for catalysts application.
are able to be synthesized using the microemulsion method at room temperature without calcination process and it is suitable for catalysts application.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cerium oxide  , otherwise known as ceria, can be found abundantly, comprises of about 66 ppm of the earth's crust, and it has been classified as a rare earth element [1]. Some of its attributes include a hint of pale yellow powder, possesses a cubic fluorite structure and its lattice parameter is
, otherwise known as ceria, can be found abundantly, comprises of about 66 ppm of the earth's crust, and it has been classified as a rare earth element [1]. Some of its attributes include a hint of pale yellow powder, possesses a cubic fluorite structure and its lattice parameter is  [2]. Ceria has been recognized as an important material for application in various fields such as ceramics [3], photocatalysts [4, 5], UV absorbers [6], catalysts [7–9] and the most interesting one is the application of ceria in medicine for anti-cancer [10]. Ceria promotes water–gas shift reaction [13], and ceria has the ability to improve the dispersion of noble metals [14, 15].
[2]. Ceria has been recognized as an important material for application in various fields such as ceramics [3], photocatalysts [4, 5], UV absorbers [6], catalysts [7–9] and the most interesting one is the application of ceria in medicine for anti-cancer [10]. Ceria promotes water–gas shift reaction [13], and ceria has the ability to improve the dispersion of noble metals [14, 15].
The characteristic that renders ceria as an effective catalyst is oxygen storage capacity (OSC) due to its ability to undergo transformation from a stoichiometric  (+4) valance state to
(+4) valance state to  (+3) state with low-energy reaction [11, 12]. Furthermore, the ability of ceria to store/release oxygen species is the most promising and efficient feature in the three-way catalysts (TWCs), which is able to control the emission of pollutant produced by motor vehicles. In motor vehicles operation, oxygen concentrations are easily fluctuating in the catalytic exhaust system. Ceria will act as oxygen storage/release, which undergoes the oxidation/reduction of
(+3) state with low-energy reaction [11, 12]. Furthermore, the ability of ceria to store/release oxygen species is the most promising and efficient feature in the three-way catalysts (TWCs), which is able to control the emission of pollutant produced by motor vehicles. In motor vehicles operation, oxygen concentrations are easily fluctuating in the catalytic exhaust system. Ceria will act as oxygen storage/release, which undergoes the oxidation/reduction of  with reversible reaction under rich or lean oxygen condition to ensure that the ratio for stoichiometry of air/fuel is met at the theoretical value of 14.6 [16]. In TWCs, ceria also acts as catalyst support and the holder of active catalysts. However, when the TWCs is exposed to high temperatures, the bond interaction of active catalysts and ceria becomes weak and leads to the sintering of the catalysts [17, 18], resulting in thermal deactivation phenomenon. The sintering phenomenon in TWCs leads to the restructuring of the surface and bulk catalysts, with pore collapse in the ceria surface, crystal growth and agglomeration of active catalysts and ceria, resulting in the reduction of active site [19]. Consequently, the redox properties and the rate of catalytic activity of ceria decrease. Some of researchers suggested that the doping of transition metals into the ceria structure is able to promote the high thermal stability and enhanced the dynamic of oxygen mobility [20, 21]. The modification of ceria with other transition metals plays a significant role in the development of efficient oxygen storage materials in TWCs.
with reversible reaction under rich or lean oxygen condition to ensure that the ratio for stoichiometry of air/fuel is met at the theoretical value of 14.6 [16]. In TWCs, ceria also acts as catalyst support and the holder of active catalysts. However, when the TWCs is exposed to high temperatures, the bond interaction of active catalysts and ceria becomes weak and leads to the sintering of the catalysts [17, 18], resulting in thermal deactivation phenomenon. The sintering phenomenon in TWCs leads to the restructuring of the surface and bulk catalysts, with pore collapse in the ceria surface, crystal growth and agglomeration of active catalysts and ceria, resulting in the reduction of active site [19]. Consequently, the redox properties and the rate of catalytic activity of ceria decrease. Some of researchers suggested that the doping of transition metals into the ceria structure is able to promote the high thermal stability and enhanced the dynamic of oxygen mobility [20, 21]. The modification of ceria with other transition metals plays a significant role in the development of efficient oxygen storage materials in TWCs.
Currently, the incorporation of zirconium oxide (zirconia,  ) into
) into  has been widely applied in TWCs to realize the thermal resistivity of ceria. Hori et al [22] reported that cerium zirconium oxide showed high thermal stability even though the sample was exposed to a high temperature of
has been widely applied in TWCs to realize the thermal resistivity of ceria. Hori et al [22] reported that cerium zirconium oxide showed high thermal stability even though the sample was exposed to a high temperature of  and this has also been reported by other authors [23–26]. Doping with zirconia, as it is alone a non-reducible oxide, makes the cerium zirconium oxide a highly reactive, thermally stable, and more reducible with elevated oxygen storage capacity (OSC) that is important for TWC applications [27]. Thus, ceria-zirconia has been acknowledged as an oxygen storage material in TWCs.
and this has also been reported by other authors [23–26]. Doping with zirconia, as it is alone a non-reducible oxide, makes the cerium zirconium oxide a highly reactive, thermally stable, and more reducible with elevated oxygen storage capacity (OSC) that is important for TWC applications [27]. Thus, ceria-zirconia has been acknowledged as an oxygen storage material in TWCs.
Ceria was mostly synthesized using conventional methods, such as co-precipitation [28–30], sol-gel [9, 31], and hydrothermal method [32]. These methods required prolonged high-temperature heating or calcination up to  . Continuous heating at high temperature can lead to the decrease of the surface area as well as poor microstructure properties [33–35]. Consequently, palladium dispersion on the entire porous system of ceria-zirconia in TWCs was low and inhomogeneous, reducing the active site and rate of oxygen storage/release [36]. Therefore, TWCs will be less effective in controlling the pollutant emitted from motor vehicles.
. Continuous heating at high temperature can lead to the decrease of the surface area as well as poor microstructure properties [33–35]. Consequently, palladium dispersion on the entire porous system of ceria-zirconia in TWCs was low and inhomogeneous, reducing the active site and rate of oxygen storage/release [36]. Therefore, TWCs will be less effective in controlling the pollutant emitted from motor vehicles.
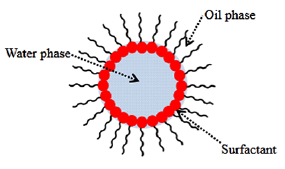
With the aim to solve these problems, the water/oil microemulsion was proposed to generate better microstructure properties, especially in producing the high surface area of oxygen storage materials. According to Schulman et al [35], who introduced the microemulsion in 1959, microemulsion was thermodynamically-stable liquid solution that contains oil, water, and amphiphile so that the surfactant is located at a certain boundary between oil and water phases. The illustration of a microemulsion droplet is presented in figure 1, which represents as reactor for reaction place of precursors and reducing agents to form metal hydroxide that is dehydrated to form a metal oxide. Meanwhile, Boutonnet et al [37] reported that the droplet size of microemulsion was approximately in the range of 6–80 nm and it was able to produce superfine particles in nanoscale. This method has succeeded in producing a larger specific area and more active sites which enhanced the catalytic activity [38]. Apart from better microstructure properties, it was also possible to control composition of mixed oxides such as intermetallic particles by reduction of metal precursor [39]. Most importantly, the reduction of metal precursors to metal oxides possibly produced the metal oxide with their original phase even without the calcination process [39–42]. The absence of calcination process in microemulsion method can avoid the formation of the poor microstructure properties in ceria zirconia due to the sintering phenomenon. In light of these developments, we have adopted microemulsion to synthesize  nanoparticles at room temperature. This work studied the structural properties of ceria-zirconia in terms of its texture and morphologies with the narrowing of size particles in the absence of calcination process. We aspire that our work may help to understand the benefits of microemulsion method to produce better properties and the relation of calcination process to the structural properties.
nanoparticles at room temperature. This work studied the structural properties of ceria-zirconia in terms of its texture and morphologies with the narrowing of size particles in the absence of calcination process. We aspire that our work may help to understand the benefits of microemulsion method to produce better properties and the relation of calcination process to the structural properties.
Figure 1. An illustration of the microemulsion droplet system.
Download figure:
Standard image High-resolution image2. Methodology
2.1. Materials
Cerium nitrate hexahydrate ( ), zirconium oxynitrate hydrate (
), zirconium oxynitrate hydrate ( ), and sodium hydroxide (NaOH) were purchased from Sigma Aldrich. As for microemulsion system, the n-octane as oil phase, the 1-butanol as co-surfactant, and the cetyltrimethylammonium bromide (CTAB) as surfactant, were purchased from Merck. Meanwhile, ethanol and distilled water for washing process were obtained from the chemistry lab, Faculty of Chemical Engineering, UiTM Shah Alam. No further purification was carried out for all chemicals and they were used as received.
), and sodium hydroxide (NaOH) were purchased from Sigma Aldrich. As for microemulsion system, the n-octane as oil phase, the 1-butanol as co-surfactant, and the cetyltrimethylammonium bromide (CTAB) as surfactant, were purchased from Merck. Meanwhile, ethanol and distilled water for washing process were obtained from the chemistry lab, Faculty of Chemical Engineering, UiTM Shah Alam. No further purification was carried out for all chemicals and they were used as received.
2.2. Preparation of nano- via microemulsion method
via microemulsion method
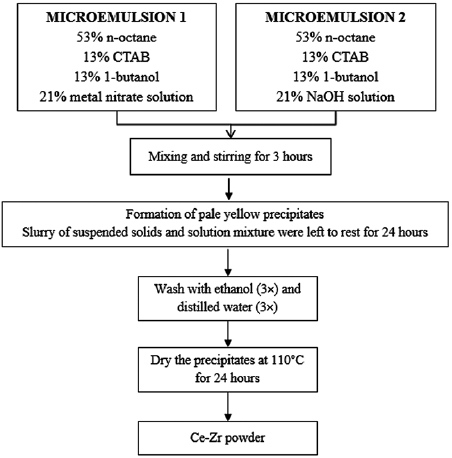
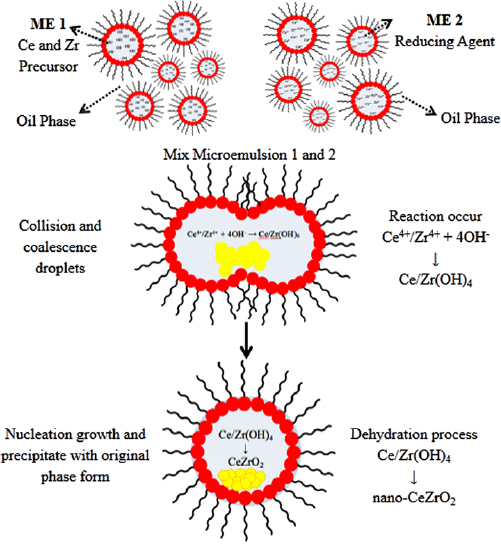
According to Malik et al [38], the microemulsion systems can be divided into two types: water/oil and oil/water systems. In this study, the water/oil system was used to synthesize the nano- particles. The synthesis procedure was adapted from Laguna et al [21] with some modifications to facilitate the route of synthesis. Two identical compositions of microemulsions were prepared with the varying aqueous solutions. Microemulsion 1 (ME 1) contained ceria and zirconia precursors, whereas the second microemulsion containing reducing agent (NaOH) was labelled as microemulsion 2 (ME 2). The synthesis route of microemulsion method was illustrated in figure 2. Microemulsion components, which were n-octane, 1-butanol, cetyltrimethylammonium bromide, and aqueous phase (water phase) were mixed under vigorous stirring until a transparent solution (stable thermodynamics) was attained. The microemulsion system was developed by the homogeneous mixing between oil, water, and surfactant to create the droplet of microemulsion or also called nanoreactor. The droplets of microemulsion contained ceria and zirconia ions for ME 1, while ME 2 contained the reducing agent. Both microemulsion systems were then mixed and continuously stirred under vigorous agitation for 3 h to ensure the formation of suspended solids, which appear in the form of pale yellow precipitates. The slurry of the suspension solids and solution mixture were left for 24 h for the dehydration process. The suspended solids were separated by centrifugation at 10,000 rpm for 10 min and washed with ethanol (3 times) and deionized (3 times) successively. The particles obtained were then dried at
particles. The synthesis procedure was adapted from Laguna et al [21] with some modifications to facilitate the route of synthesis. Two identical compositions of microemulsions were prepared with the varying aqueous solutions. Microemulsion 1 (ME 1) contained ceria and zirconia precursors, whereas the second microemulsion containing reducing agent (NaOH) was labelled as microemulsion 2 (ME 2). The synthesis route of microemulsion method was illustrated in figure 2. Microemulsion components, which were n-octane, 1-butanol, cetyltrimethylammonium bromide, and aqueous phase (water phase) were mixed under vigorous stirring until a transparent solution (stable thermodynamics) was attained. The microemulsion system was developed by the homogeneous mixing between oil, water, and surfactant to create the droplet of microemulsion or also called nanoreactor. The droplets of microemulsion contained ceria and zirconia ions for ME 1, while ME 2 contained the reducing agent. Both microemulsion systems were then mixed and continuously stirred under vigorous agitation for 3 h to ensure the formation of suspended solids, which appear in the form of pale yellow precipitates. The slurry of the suspension solids and solution mixture were left for 24 h for the dehydration process. The suspended solids were separated by centrifugation at 10,000 rpm for 10 min and washed with ethanol (3 times) and deionized (3 times) successively. The particles obtained were then dried at  for 24 h.
for 24 h.
Figure 2. Flow chart of the synthesis route of microemulsion method.
Download figure:
Standard image High-resolution image2.3. Characterization of nano- particles
particles
Dynamic light scattering (Malvern Zeta Sizer Nano-ZS) was used to identify the micelle size of droplet microemulsion, with the octane as dispersion solution and aqueous precursors solution as materials. The hydrodynamic diameter or micelle size microemulsion droplets were calculated from the Stokes-Einstein equation. X-ray diffraction (XRD, Rigaku Ultima IV) was carried out to analyze the phase and the crystallinity of the samples. The scans were taken within a range  of
of  to
to  using
using  (0.15418 nm) with a scanning rate of
(0.15418 nm) with a scanning rate of  . The crystallite size was calculated using Scherrer equation, with reference to the main intense peak (111). The phase transformation of the ceria-zirconia was studied by differential scanning calorimetric analysis (Mettler Toledo, model: DSC 822). A small number of samples were loaded in an aluminium crucible and heated from room temperature up to
. The crystallite size was calculated using Scherrer equation, with reference to the main intense peak (111). The phase transformation of the ceria-zirconia was studied by differential scanning calorimetric analysis (Mettler Toledo, model: DSC 822). A small number of samples were loaded in an aluminium crucible and heated from room temperature up to  with a heating rate of
with a heating rate of  under an air flow of 30 ml min−1. On top of that, the surface morphologies and microstructure properties were also examined using field emission scanning electron microscopy (DSM 982 Gemini Supra 40 VP) and high-resolution transmission electron microscopy (Tecnai G2 20). In meantime, the calculated d-spacing of samples was obtained from bright diffraction ring in transmission electron microscopy analysis, and then compared with the diffraction database (from XRD) to identify the plane
under an air flow of 30 ml min−1. On top of that, the surface morphologies and microstructure properties were also examined using field emission scanning electron microscopy (DSM 982 Gemini Supra 40 VP) and high-resolution transmission electron microscopy (Tecnai G2 20). In meantime, the calculated d-spacing of samples was obtained from bright diffraction ring in transmission electron microscopy analysis, and then compared with the diffraction database (from XRD) to identify the plane  of samples. Lastly, the textural properties such as the specific surface area, the average pore size, and the pore volume of the samples were studied using
of samples. Lastly, the textural properties such as the specific surface area, the average pore size, and the pore volume of the samples were studied using  adsorption-desorption (Autosorb-1 Quanta Chrome Instrument TUSA).
adsorption-desorption (Autosorb-1 Quanta Chrome Instrument TUSA).
3. Results and discussion
3.1. Formation of nano- particle using microemulsion method
particle using microemulsion method
The formation of nano- particle using microemulsion method usually followed a typical synthesis procedure with the mixing of two microemulsions, one of which contained the cation and another microemulsion contained the reducing agents. The cations used in this study are the ceria and zirconia ions and the reducing agents is the sodium hydroxide which promotes the hydroxide ions. The cation and anion are present inside the droplet of microemulsion or micelle for nucleation reaction. A mechanism of
particle using microemulsion method usually followed a typical synthesis procedure with the mixing of two microemulsions, one of which contained the cation and another microemulsion contained the reducing agents. The cations used in this study are the ceria and zirconia ions and the reducing agents is the sodium hydroxide which promotes the hydroxide ions. The cation and anion are present inside the droplet of microemulsion or micelle for nucleation reaction. A mechanism of  nanoparticle formation during synthesis route is shown in figure 3. In which the ME 1 which contains the ceria-zirconia ions is added into the ME 2 which contains the hydroxide ions. Then, the mixing of both MEs under vigorous stirring produces Brownian motion, which leads to the collision and the coalescence of droplets. Then, the nucleation between ceria-zirconia ions and hydroxide ions occurs inside the droplets, and the particles begin to form and grow during the collision and the coalescence of the droplets under vigorous agitation. The surfactant prevents the rapid growths of particles by controlling their particle size and producing a homogenous size distribution. This mechanism is adopted from Capek et al [41].
nanoparticle formation during synthesis route is shown in figure 3. In which the ME 1 which contains the ceria-zirconia ions is added into the ME 2 which contains the hydroxide ions. Then, the mixing of both MEs under vigorous stirring produces Brownian motion, which leads to the collision and the coalescence of droplets. Then, the nucleation between ceria-zirconia ions and hydroxide ions occurs inside the droplets, and the particles begin to form and grow during the collision and the coalescence of the droplets under vigorous agitation. The surfactant prevents the rapid growths of particles by controlling their particle size and producing a homogenous size distribution. This mechanism is adopted from Capek et al [41].
Figure 3. Mechanism of synthesis routes in microemulsion method.
Download figure:
Standard image High-resolution imageDuring the experiment, it can be observed that the colour of mixture of both MEs continuously changed from dark grey to pale yellow, as shown in figure 4. Initially, the cerium and zirconium solution at 0.75 M was added to the milky solution of the mixture of CTAB, butanol and octane as the basic component to form the droplet of microemulsion. Then, the milky solution turned to clear solution because the mixing of oil and water attained the thermodynamic stability, as reported by Boutonnet et al [37]. Then, two MEs were mixed. Interestingly, the colour of the solution changed from colourless to dark brown, and then simultaneously changed to pale yellow after 15 min of stirring. Then, no further colour change was observed by continuing the stirring up to 3 h. It indicates that the colour change is due to the occurrence of some chemical reactions in the ME system. Cerium and zirconium ions reacted with the hydroxide ions inside the droplet of microemulsions to produce the cerium hydroxide and zirconium hydroxide. This finding is similar to the report by Wang and Dangerfield [43], which focused on the synthesis of cerium oxide, found that the colour change from the dark grey to pale yellow during the precipitation of cerium oxide is due to the precipitation and oxidation of cerium. They claimed that the cerium ions react with the hydroxide ions to form the cerium hydroxide precipitates (dark grey) which are unstable in air even at the room temperature. Then cerium hydroxide turned to  (pale yellow) during the oxidation or dehydration reaction with the presence of oxygen during vigorous stirring in air. This explanation is consistent with our observation as shown in figure 4, the colour of ME solution changed from dark grey to pale yellow in the first 15 min. This indicates the occurrence of precipitation and oxidation processes inside the droplet of microemulsion to produce the cerium and zirconium oxides. The chemical reactions of the processes are as follows:
(pale yellow) during the oxidation or dehydration reaction with the presence of oxygen during vigorous stirring in air. This explanation is consistent with our observation as shown in figure 4, the colour of ME solution changed from dark grey to pale yellow in the first 15 min. This indicates the occurrence of precipitation and oxidation processes inside the droplet of microemulsion to produce the cerium and zirconium oxides. The chemical reactions of the processes are as follows:
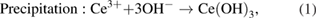
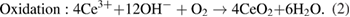
Figure 4. Colour change of suspension in the microemulsion during stirring.
Download figure:
Standard image High-resolution imageIn addition, a small number of zirconium ions available in the ME will also react to form the zirconium hydroxide, but it may not dehydrate to be a zirconium oxide because the transformation of zirconium hydroxide required some energy. Hence, the  could be a crystallite and the zirconium hydroxide naturally in amorphous phase [44]. Some modifications on the synthesis route in this method was made, in which the precipitated ceria-zirconia was aged for 24 h and underwent drying process at
could be a crystallite and the zirconium hydroxide naturally in amorphous phase [44]. Some modifications on the synthesis route in this method was made, in which the precipitated ceria-zirconia was aged for 24 h and underwent drying process at  for 24 h which is to ensure that all metal hydroxides will be transformed into metal oxide, especially the cerium. Therefore, it can be concluded that the colour change of suspension in the microemulsions during stirring is consistent with the mechanism of microemulsion as reported by others author [21, 37, 41].
for 24 h which is to ensure that all metal hydroxides will be transformed into metal oxide, especially the cerium. Therefore, it can be concluded that the colour change of suspension in the microemulsions during stirring is consistent with the mechanism of microemulsion as reported by others author [21, 37, 41].
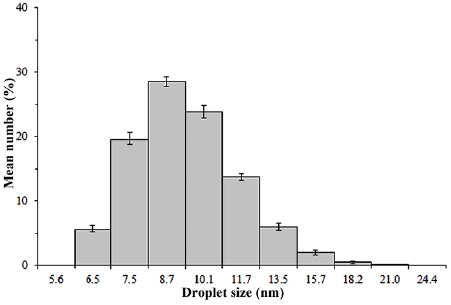
3.2. Distribution of microemulsion droplet size
Figure 5 depicts the distribution of droplet size of the microemulsion measured using Malvern zeta sizer nano-ZS. The droplets are distributed in narrow nanometre scale, in the range of 6.5 to 21 nm. The most dominant droplet size of the microemulsion system is about 8.7 nm. As described in the mechanism of microemulsion, the introduction of surfactant will help in preventing the rapid growth of particles, resulting in the formation of nanosize particles as big as the size of microemulsion droplet [41]. It indicates that the microemulsion system is able to produce metal oxides particles in nanosize scale. The technique is also able to generate uniform and controlled-size distribution of metal oxides particles, as shown in figure 5.
Figure 5. Distribution of microemulsion droplet size.
Download figure:
Standard image High-resolution image3.3. Structure and crystallinity investigation
In order to investigate the microstructure transformation, two synthesized ceria-zirconia samples were treated under two different conditions. The first sample had undergone calcination process, while another sample without calcination process. XRD patterns for both samples are portrayed in figure 6. The XRD patterns for both samples show typical reflections of a cubic structure of cerium zirconium oxide,  (ICDD file: 01-074-8064), in which the characteristic peaks identified at angle
(ICDD file: 01-074-8064), in which the characteristic peaks identified at angle  of 28.79, 33.36, 47.90, 56.85, and 59.63 are assigned to planes (111), (200), (220), (311), and (222), respectively.
of 28.79, 33.36, 47.90, 56.85, and 59.63 are assigned to planes (111), (200), (220), (311), and (222), respectively.
Figure 6. XRD patterns of ceria-zirconia samples; (a) without calcination, and (b) with calcination process.
Download figure:
Standard image High-resolution imageFor sample without calcination process, a noise line with the broader peaks is observed (figure 6(b)). The peaks (200) and (222) do not clearly appear in the pattern, due to the broadening of peaks (111) and (311). In contrast, the calcination sample shows a smoother line, sharper peaks, and intense peaks, indicating high crystallinity of samples (figure 6(b)). The result is consistent with the report by Li et al [24]. In addition, Liu et al [4] also reported that the sharp and strong peaks indicate the good crystallization of the samples. In other words, when the peaks of diffractogram become sharper and intense, it indicates the growth of the crystallinity. From this result, it can be concluded that the sample without calcination has crystalline and amorphous phases.
Table 1 presents the comparison of peak-to-noise ratio from XRD pattern. It seems that ceria-zirconia sample without calcination process has lower peak-to-noise ratio compared to the ceria-zirconia sample with calcination process, indicating that the ceria-zirconia without calcination process is naturally formed as amorphous phase. The finding is consistent with the past studies by Lopez and Mendoza [45], which reported that the nanoparticles of cerium oxide produced by microemulsion method are become amorphous in nature. In other report, Hadi and Yaacob [39] found that the synthesized cerium oxide via microemulsion method without calcination process also reveals a well-crystalline structure. Therefore, the availability of the crystalline phase in without calcination samples synthesized by microemulsion is possible and predictable. As described by Callister [46], the atoms in crystallite materials are positioned in orderly and repeated patterns that are in contrast to the random and disordered atomic distribution found in amorphous materials. In ceria-zirconia system, the presence of zirconia ions inside the ceria structure produces the structure defects with the disordered atomic arrangement and it leads to the transformation of precursor's ceria-zirconia into the amorphous structure in nature. In terms of crystallographic principle, the unstable  crystal structure extremely improved the OSC value as well as the ability of transforming the
crystal structure extremely improved the OSC value as well as the ability of transforming the  into
into  , respectively as concluded by Min et al [47]. The similar finding of ceria-zirconia synthesized using microemulsion method that also produced an amorphous material was reported by Masui et al [25]. Therefore, it is safe to say that the phases of ceria-zirconia sample without calcination process are the mixture of amorphous and crystalline nanoparticles.
, respectively as concluded by Min et al [47]. The similar finding of ceria-zirconia synthesized using microemulsion method that also produced an amorphous material was reported by Masui et al [25]. Therefore, it is safe to say that the phases of ceria-zirconia sample without calcination process are the mixture of amorphous and crystalline nanoparticles.
Table 1. Phase and crystallinity of ceria-zirconia; (a) without calcination, and (b) with calcination process.
| Sample | Phase | Angle 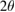 main peak (111) main peak (111) |
Lattice parameter (nm) | Crystallite size (nm) | Peak-to-noise ratio (P/N) |
|---|---|---|---|---|---|
| a | Cubic | 28.32 | 0.5454 | 9 | 1.8 |
| b | Cubic | 28.62 | 0.5398 | 11.5 | 9.7 |
| ICDD file 01-074-8064 | Cubic | 28.79 | 0.5367 | — | — |
| ICDD file 00-002-1306 | Cubic | 28.56 | 0.5411 | — | — |
Nevertheless, no tetragonal peaks or unidentified peaks are observed in both patterns, indicating the formation of complete solid solution. The complete insertion of zirconia ions into the ceria lattice does not display the phase segregation and only a cubic phase structure is formed. This fact may elaborate on formation of solid solution. The smaller radius ions of zirconia (0.084 nm) easily solute into the larger ions radii of ceria lattice (0.097 nm) and the crystal structure is, therefore, maintained as ceria phase [24]. There is no the segregation phase found, in which the ceria and zirconia are separately formed as individual phase. Teng et al [48] also stated that the broadening of peak in the diffractogram may be defined as the symmetry of solid solution phases or the co-presence of some amount of tetragonal phases in Ce-rich materials. In addition, the precursors of ceria and zirconia peaks were not detected, also indicating complete reaction of ceria-zirconia with the precipitating agents inside the microemulsion droplet. According to Capek [41], the microemulsion droplet acts as nanoreactor where in the nucleation reactions between cation (ceria and zirconia) and anion (reducing agents) take place to form metal hydroxide, which is then dehydrated during aging process to form metal oxide. Therefore, the microemulsion method possibly produced the homogeneous phase of cerium-zirconium mixed-oxide.
As comparison, the ICDD file of crystalline cerium oxide,  (ICDD file: 021306) is also tabulated in table 1. The insertion of Zr into
(ICDD file: 021306) is also tabulated in table 1. The insertion of Zr into  lattice structure shifts the angle towards higher angle of main peak (111) and it is identified as the displacement of the diffraction angle
lattice structure shifts the angle towards higher angle of main peak (111) and it is identified as the displacement of the diffraction angle 
 to
to  , for
, for  with calcination. This shifting relates to the phase transformation and the formation of cubic structure in ceria-zirconia mixture. Meanwhile, the expansion of lattice parameter is also observed in
with calcination. This shifting relates to the phase transformation and the formation of cubic structure in ceria-zirconia mixture. Meanwhile, the expansion of lattice parameter is also observed in  and synthesized
and synthesized  with calcination, which indicates the influences of zirconium in ceria structure. It is due to the effect of interstitial solid solution of smaller Zr ions into the Ce structure. The insertion of the small radius ions
with calcination, which indicates the influences of zirconium in ceria structure. It is due to the effect of interstitial solid solution of smaller Zr ions into the Ce structure. The insertion of the small radius ions  (0.087 nm) into the bigger radius ions
(0.087 nm) into the bigger radius ions  (0.097 nm) causes the increased of the lattice parameter and the shifting angle of main peak (111).
(0.097 nm) causes the increased of the lattice parameter and the shifting angle of main peak (111).
Other than that, the shifting angle  for peak (111) to the right is due to the rearrangement of the atom order during crystallization process, which transforms amorphous to crystallite phase of ceria-zirconia. The similar finding is reported by Lopez and Mendoza [45]. In consistent findings, the effect of calcination process can be explained through study carried out by Biswas and Bandyopadhyay on Nd-doped ceria, which revealed the transformation of an unstable phase of Nd-Ceria solid solution to stable cubic phases during crystallization process. The absorbed water is removed at temperature of
for peak (111) to the right is due to the rearrangement of the atom order during crystallization process, which transforms amorphous to crystallite phase of ceria-zirconia. The similar finding is reported by Lopez and Mendoza [45]. In consistent findings, the effect of calcination process can be explained through study carried out by Biswas and Bandyopadhyay on Nd-doped ceria, which revealed the transformation of an unstable phase of Nd-Ceria solid solution to stable cubic phases during crystallization process. The absorbed water is removed at temperature of  [49]. Therefore, it can be concluded that the shifting
[49]. Therefore, it can be concluded that the shifting  value of main peaks from 28.32 to 28.62 is due to the expansion of cubic structure of ceria by the transformation of amorphous to crystallite phase.
value of main peaks from 28.32 to 28.62 is due to the expansion of cubic structure of ceria by the transformation of amorphous to crystallite phase.
However, the  without calcination may not be fully formed as interstitial or substitution solid solution, indicated by the shifting angle to the left if it is compared to ICDD file of
without calcination may not be fully formed as interstitial or substitution solid solution, indicated by the shifting angle to the left if it is compared to ICDD file of  . But, the lattice parameter of synthesized
. But, the lattice parameter of synthesized  without calcination is bigger than that of
without calcination is bigger than that of  and
and  with calcination. It is predicted that the structure of
with calcination. It is predicted that the structure of  without calcination consists of the amorphous phase, which is the cerium hydroxide. In other word, cerium hydroxide was not fully converted during calcination process.
without calcination consists of the amorphous phase, which is the cerium hydroxide. In other word, cerium hydroxide was not fully converted during calcination process.
The ceria-zirconia samples without calcination process reveals the broadening of peak (111) and lower intensity peak than that of ceria-zirconia samples with calcination process. The width of the main peak indicates the greatness and the smallness of the crystallite size. The peaks with narrow width are attributed to huge crystallite size and conversely [39, 50–52]. These findings revealed that the crystallite size of ceria-zirconia without calcination process was smaller than that of the ceria-zirconia samples with calcination process. The growth of crystal size of ceria-zirconia was explained by Simonsen et al [53] and Pamu et al [54], in which the calcination process of ceria-zirconia allowed the Ostwald ripening phenomenon to occur, whereby the smaller crystal species will diffuse from smaller to larger crystal. Xiao et al [55] also stated that the high migration of ions between the transformations of  to
to  species under high temperature may cause grain growth or sintering and also the oxygen vacancies are formed. Therefore, the larger particles eventually grow at the expense of the smaller ones. This fact is consistent with the data pertaining to crystallite size for both samples in table 1. The crystallite size of ceria-zirconia without calcination process is 9 nm, while the ceria-zirconia samples that undergo the calcination process rapidly grow up to 11.5 nm. Several studies also reported the effect of calcination process of metal oxide on the increase of the size of crystal and enhanced the construction to large clusters [33, 34, 56].
species under high temperature may cause grain growth or sintering and also the oxygen vacancies are formed. Therefore, the larger particles eventually grow at the expense of the smaller ones. This fact is consistent with the data pertaining to crystallite size for both samples in table 1. The crystallite size of ceria-zirconia without calcination process is 9 nm, while the ceria-zirconia samples that undergo the calcination process rapidly grow up to 11.5 nm. Several studies also reported the effect of calcination process of metal oxide on the increase of the size of crystal and enhanced the construction to large clusters [33, 34, 56].
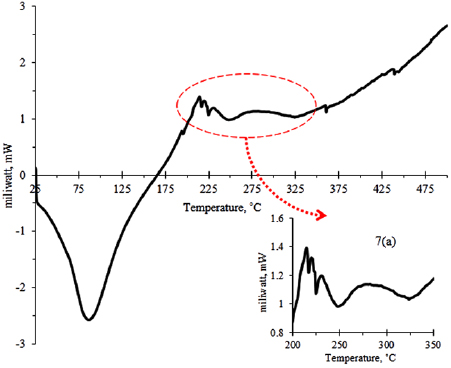
3.4. Phase transformation of ceria-zirconia
Figure 7 reveals the DSC profile of ceria-zirconia without calcination process with constants heating rate of  in air. A similar condition of calcination process is implemented in the DSC analysis to study the phase transformation of amorphous to crystallite ceria-zirconia. A few exothermic and endothermic peaks were observed in figure 7. A major endothermic peak at
in air. A similar condition of calcination process is implemented in the DSC analysis to study the phase transformation of amorphous to crystallite ceria-zirconia. A few exothermic and endothermic peaks were observed in figure 7. A major endothermic peak at  is due to the evaporation of absorbed water and the volatile compound in the microemulsion system. A major exothermic peak at range temperature of
is due to the evaporation of absorbed water and the volatile compound in the microemulsion system. A major exothermic peak at range temperature of  corresponds to the dehydration of ceria-zirconia hydroxide in formation of metal oxide under unstable phase. The similar finding was reported by Biswas and Bandyopadhyay [49], and Kumar and Mathews [57]. As seen in figure 7(a), the endothermic peak at
corresponds to the dehydration of ceria-zirconia hydroxide in formation of metal oxide under unstable phase. The similar finding was reported by Biswas and Bandyopadhyay [49], and Kumar and Mathews [57]. As seen in figure 7(a), the endothermic peak at  is attributed to the crystallization of cerium oxide. Regarding the calcination temperature required to form crystalline structure of
is attributed to the crystallization of cerium oxide. Regarding the calcination temperature required to form crystalline structure of  , Kumar and Mathews [57] also reported that the DSC analysis of pure cerium oxide presents a sharp peak at temperature of
, Kumar and Mathews [57] also reported that the DSC analysis of pure cerium oxide presents a sharp peak at temperature of  without weight loss detected and it may corresponds to the crystallization of cerium oxide, while the transformation of amorphous to crystallite phase of zirconia occurs above
without weight loss detected and it may corresponds to the crystallization of cerium oxide, while the transformation of amorphous to crystallite phase of zirconia occurs above  . Besides, they also found that the transformation of amorphous to crystallite ceria-zirconia occurs at above
. Besides, they also found that the transformation of amorphous to crystallite ceria-zirconia occurs at above  . In this work, the minor endothermic peak at
. In this work, the minor endothermic peak at  in
in  may correspond to the crystallization peak of zirconium oxide. However, in this study, the crystallization peak did not clearly appear, possibly because the conversion of amorphous to crystalline phase is too small. Besides, the previous work by Hadi and Yaacob [39] also found that well-crystallized cerium oxide is successfully prepared by microemulsion method in room temperature without calcination process. It indicates that the semi-crystallite of
may correspond to the crystallization peak of zirconium oxide. However, in this study, the crystallization peak did not clearly appear, possibly because the conversion of amorphous to crystalline phase is too small. Besides, the previous work by Hadi and Yaacob [39] also found that well-crystallized cerium oxide is successfully prepared by microemulsion method in room temperature without calcination process. It indicates that the semi-crystallite of  is due to the insertion of Zr in ceria structure, which required more energy to crystallize. This finding proves that the synthesized ceria-zirconia without calcination process still produces a crystallite structure although it is not fully crystallized and transformed as semi-crystallite in nature. This result is also supported by XRD findings as previously discussed.
is due to the insertion of Zr in ceria structure, which required more energy to crystallize. This finding proves that the synthesized ceria-zirconia without calcination process still produces a crystallite structure although it is not fully crystallized and transformed as semi-crystallite in nature. This result is also supported by XRD findings as previously discussed.
Figure 7. DSC profile of synthesized ceria-zirconia without calcination process.
Download figure:
Standard image High-resolution image3.5. Morphology properties
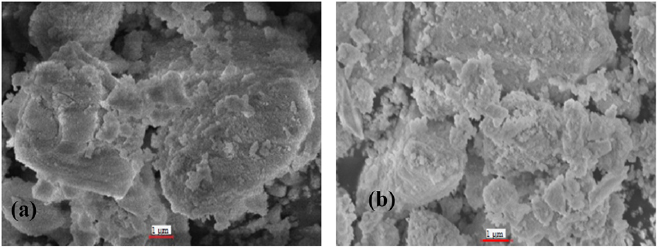
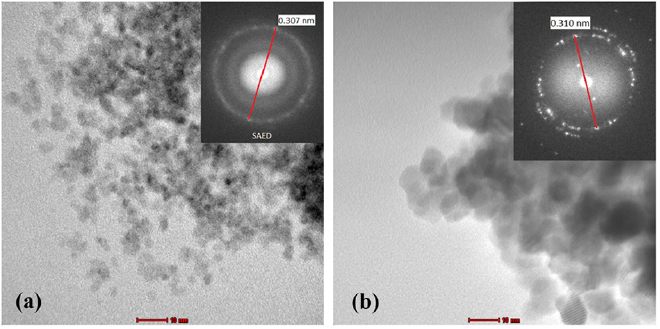
The surface morphologies for both samples are displayed in figure 8. The micrograph for both samples shows the symmetrical surface morphology even though the sample in figure 8(b) without calcination process. It indicated that the microemulsion technique could produce a uniform morphology property even though the calcination process was not implemented. Further investigation, the transmission electron microscopy analysis was conducted to determine the morphology properties of both samples. Figure 9 shows the micrographs of transmission electron microscopy for both samples. The spherical shape for both samples was observed.
Figure 8. SEM micrographs of ceria-zirconia; (a) without calcination, and (b) with calcination process.
Download figure:
Standard image High-resolution imageFigure 9. TEM micrographs of ceria-zirconia; (a) without calcination and (b) with calcination process.
Download figure:
Standard image High-resolution imageThe size of ceria-zirconia particles that underwent the calcination process was found to be bigger than that of the sample without calcination process. On top of that, particle size for ceria-zirconia undergoing the calcination process was distributed in the range of 10–12 nm, while the sample without calcination process was in the range  . It indicated that the calcination process led to the collapse of crystal, its growth, as well as agglomeration, resulting in the increase of particles size. The previous work by Chen et al [34] also reported that the crystallization of
. It indicated that the calcination process led to the collapse of crystal, its growth, as well as agglomeration, resulting in the increase of particles size. The previous work by Chen et al [34] also reported that the crystallization of  could be at room temperature in natural conversion during precipitation by addition of reducing agents. The crystal size of
could be at room temperature in natural conversion during precipitation by addition of reducing agents. The crystal size of  further increased from 12 nm to 48 nm as calcined from 200 °C to
further increased from 12 nm to 48 nm as calcined from 200 °C to  . In different materials, the development of
. In different materials, the development of  also faced the similar issue as reported by Gaber et al [56]. In which, the crystallite size increased from 3.45 nm to 23.50 nm as calcination temperature increased from
also faced the similar issue as reported by Gaber et al [56]. In which, the crystallite size increased from 3.45 nm to 23.50 nm as calcination temperature increased from  to
to  . The enlargement of crystallite size may relate to the Ostwald ripening phenomenon, whereby the smaller crystal easily diffused to larger crystal and larger crystal eventually grew at the expense of the smaller ones. In which the particle size of ceria-zirconia samples in droplet microemulsion possessed a narrow size and it diffused to larger crystal as well as the enlargement of crystal continuously. Based on this finding, we reveal that the droplets are able to control the growth of particle size within the boundary of surfactant during the nucleation reactions between ceria, zirconia, and reducing agents, which produced the particles with size within the range of droplet size of microemulsion.
. The enlargement of crystallite size may relate to the Ostwald ripening phenomenon, whereby the smaller crystal easily diffused to larger crystal and larger crystal eventually grew at the expense of the smaller ones. In which the particle size of ceria-zirconia samples in droplet microemulsion possessed a narrow size and it diffused to larger crystal as well as the enlargement of crystal continuously. Based on this finding, we reveal that the droplets are able to control the growth of particle size within the boundary of surfactant during the nucleation reactions between ceria, zirconia, and reducing agents, which produced the particles with size within the range of droplet size of microemulsion.
Furthermore, the micrographs of ceria-zirconia with and without calcination processes also reveal the bright diffraction rings as shown in figure 9. The calculated  spacing from bright diffraction ring in selected area electron diffraction for both samples showed the similar
spacing from bright diffraction ring in selected area electron diffraction for both samples showed the similar  spacing, which are 0.310 nm for calcination sample and 0.307 nm for without calcination sample. Comparing the
spacing, which are 0.310 nm for calcination sample and 0.307 nm for without calcination sample. Comparing the  spacing value of the sample with the XRD database of ceria-zirconia (ICDD file of 021360) which is 0.311 nm corresponding to plane (111), it was found that the error percentage of
spacing value of the sample with the XRD database of ceria-zirconia (ICDD file of 021360) which is 0.311 nm corresponding to plane (111), it was found that the error percentage of  spacing for each sample was <1%, indicating that both samples were in crystallite structure. Interestingly, this result found that the crystallite structure of ceria-zirconia can be produced using microemulsion method without calcination process. It can also be concluded that the noise in the diffraction pattern from XRD data, as shown in figure 6(a), was caused by the lower degree of crystallinity or the small particles. Furthermore, as described by Callister [46], crystalline materials reflect orderly-positioned atoms and repeated patterns, while random and disordered atomic distribution is found in amorphous materials. This indicated that a few modifications at dehydration process during preparation of sample, whereby the samples were aged for 24 h at room temperature and dried at
spacing for each sample was <1%, indicating that both samples were in crystallite structure. Interestingly, this result found that the crystallite structure of ceria-zirconia can be produced using microemulsion method without calcination process. It can also be concluded that the noise in the diffraction pattern from XRD data, as shown in figure 6(a), was caused by the lower degree of crystallinity or the small particles. Furthermore, as described by Callister [46], crystalline materials reflect orderly-positioned atoms and repeated patterns, while random and disordered atomic distribution is found in amorphous materials. This indicated that a few modifications at dehydration process during preparation of sample, whereby the samples were aged for 24 h at room temperature and dried at  , were able to facilitate the transformation of random atomic distribution to orderly-positioned distribution and consequently the revolution of amorphous phase to semi-crystalline structure. Based on the findings, it is found that the microemulsion method is able to produce metal oxide particles with their original phase at room temperature without calcination process.
, were able to facilitate the transformation of random atomic distribution to orderly-positioned distribution and consequently the revolution of amorphous phase to semi-crystalline structure. Based on the findings, it is found that the microemulsion method is able to produce metal oxide particles with their original phase at room temperature without calcination process.
3.6. Textural properties
Generally, the catalysts activity is a vital factor in a catalyst, which should be as high as possible to promote chemical reaction. According to Ichinose et al [40], the activity of a solid catalyst is proportional to the surface area, whereby particles with smaller size provided greater surface area and resulted in the enhancement of the number of active sites. In addition, particles with narrow size provided greater irregularity in the surface and enhanced the distribution of catalysts in the entire surface of catalyst support, resulting in higher space-time yield rate of the reaction device. Hence, the surface area is a vital factor to enhance the catalytic performance of catalysts.
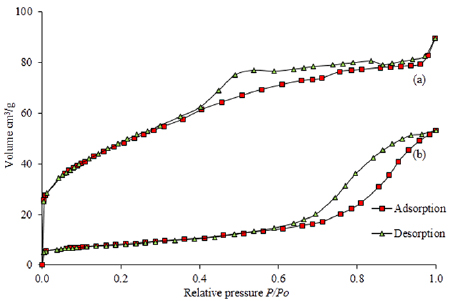
Figure 10 presents the nitrogen gas adsorption-desorption isotherm profile for the synthesized ceria-zirconia samples. The isotherm profile of ceria-zirconia with calcination process depicts the type IV isotherm in accordance with IUPAC classification (mesoporous) as shown in figure 10(b). On the other hand, the isotherm profile for without calcination sample in figure 10(a) reveals a well-defined plateau and is classified as Type I in accordance with IUPAC classification (microporous). The primary adsorption in the low relative pressure (less than 0.1) with a steep increase of absorbed nitrogen gas indicates the formation of high microporous materials with the possibility of narrow pore size distribution [58]. In this study, the sample of ceria-zirconia without calcination presents higher absorption of nitrogen gas compared to the calcination ceria-zirconia sample. This finding suggests that the ceria-zirconia without calcination produced high possibility of narrow pore size distribution.
Figure 10. Isotherm profile of ceria-zirconia; (a) without calcination, and (b) with calcination process.
Download figure:
Standard image High-resolution imageThe slightly enhanced uptake of  at
at  in the range of 0.1–0.9 suggested that it also had marginal quantity of mesopores and more uptakes of
in the range of 0.1–0.9 suggested that it also had marginal quantity of mesopores and more uptakes of  were present in a small quantity of macropores. This observation implied that the calcination ceria-zirconia sample had higher possibility in displaying the appearance of mesopores due to the phenomenon of collapse of the micropores during the calcination process, resulting in the increment of size in the pores. The formation of hysteresis in the isotherm profile is due to the availability of pore mouth or attributed to the pore size. Hence, the isotherms for both samples showed a significant degree of hysteresis, which was attributed to the presence of mesoporous structures. These findings might suggest that the formation of hysteresis for the without calcination samples was because ceria-zirconia samples without calcination process had been due to the presence of mesoporous structure, and it could be possible that the implementation of calcination process at higher temperature caused the collapse of mesopores to macropores [56]. Meanwhile, the hysteresis isotherm of without calcination sample seemed to display the reversible process of adsorption-desorption, indicating that the structure was robust and had permanent porosity, which did not require any calcination process. Therefore, the synthesis of ceria-zirconia without calcination process exhibited better textural properties, especially the narrow pore sized distribution and formation of hysteresis, which was attributed to the good adsorption-desorption phenomenon.
were present in a small quantity of macropores. This observation implied that the calcination ceria-zirconia sample had higher possibility in displaying the appearance of mesopores due to the phenomenon of collapse of the micropores during the calcination process, resulting in the increment of size in the pores. The formation of hysteresis in the isotherm profile is due to the availability of pore mouth or attributed to the pore size. Hence, the isotherms for both samples showed a significant degree of hysteresis, which was attributed to the presence of mesoporous structures. These findings might suggest that the formation of hysteresis for the without calcination samples was because ceria-zirconia samples without calcination process had been due to the presence of mesoporous structure, and it could be possible that the implementation of calcination process at higher temperature caused the collapse of mesopores to macropores [56]. Meanwhile, the hysteresis isotherm of without calcination sample seemed to display the reversible process of adsorption-desorption, indicating that the structure was robust and had permanent porosity, which did not require any calcination process. Therefore, the synthesis of ceria-zirconia without calcination process exhibited better textural properties, especially the narrow pore sized distribution and formation of hysteresis, which was attributed to the good adsorption-desorption phenomenon.
On top of that, the textural properties of samples, such as pore size, pore volume, and specific surface area were listed in table 2. The without calcination ceria-zirconia sample showed the higher surface area compared to the calcination ceria-zirconia sample. It also reveals that the calcination process at high temperature reduced the specific surface area and enlarged the pore size. These results are consistent with our previous study on the relationship between calcination and pore sizes [39, 52]. The promising characteristics of ceria-zirconia produced, which are higher surface area, good adsorption, and narrow pore size distribution, leads to the enhancement of catalytic activity.
Table 2. The surface area properties of ceria-zirconia: (a) without calcination, and (b) with calcination process.
| Sample | Surface area, 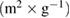 |
Pore size (nm) | Pore volume 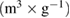 |
|---|---|---|---|
| a | 169.80 | 3.27 | 0.139 |
| b | 46.59 | 11.28 | 0.083 |
4. Conclusions
Semi-crystalline ceria-zirconia,  has been successfully produced using water/oil microemulsion method at room temperature without calcination process. Consistencies of the crystal size, particle and the droplet size of microemulsion were obtained at 8.7 nm, 9 nm, and
has been successfully produced using water/oil microemulsion method at room temperature without calcination process. Consistencies of the crystal size, particle and the droplet size of microemulsion were obtained at 8.7 nm, 9 nm, and  , respectively. The incorporation of zirconia ions into the ceria possess formed a cubic fluorite structure and solid solution. High surface area of semi-crystalline ceria-zirconia up to
, respectively. The incorporation of zirconia ions into the ceria possess formed a cubic fluorite structure and solid solution. High surface area of semi-crystalline ceria-zirconia up to  was obtained. The pore size and pore volume was also obtained at 3.27 nm and
was obtained. The pore size and pore volume was also obtained at 3.27 nm and  , respectively. The isotherm profile of semi-crystalline ceria-zirconia showed a highly microporous material (type 1) according the IUPAC standard and the hysteresis also showed the availability of mesoporous surface. Ceria-zirconia which underwent the calcination process showed a well-crystalline structure but the growth of crystal and particle size up to 11.5 nm and 12 nm, respectively. And also, the surface area was reduced to
, respectively. The isotherm profile of semi-crystalline ceria-zirconia showed a highly microporous material (type 1) according the IUPAC standard and the hysteresis also showed the availability of mesoporous surface. Ceria-zirconia which underwent the calcination process showed a well-crystalline structure but the growth of crystal and particle size up to 11.5 nm and 12 nm, respectively. And also, the surface area was reduced to  . Therefore, the semi-crystalline nano-
. Therefore, the semi-crystalline nano- could be synthesized using microemulsion method at room temperature without calcination process and it offers the better properties of ceria-zirconia which are suitable in catalyst application.
could be synthesized using microemulsion method at room temperature without calcination process and it offers the better properties of ceria-zirconia which are suitable in catalyst application.
Acknowledgments
The authors would like to acknowledge e-Science Project No. 06-01-01-SF0502 supported by Ministry of Science Technology and Innovation (MOSTI), Malaysia. We would like to thank Universiti Teknologi MARA (UiTM) for supporting the research.