Abstract
This study presents a high porous nanocomposite composed of reduced graphene oxide and NiMoS4 (RGO/NiMoS4) for supercapacitor electrode materials. The different morphologies of NiMoS4 growth on RGO sheets were obtained via a facile one-step microwave-assisted method. The obtained material comprised NiMoS4 nanoneedles of several nanometers in diameter, which are well attached to the surface of RGO nanosheets. The RGO/NiMoS4 nanocomposite had a mesoporous structure, high specific surface area, and high electric conductivity. These excellent characteristics yield a fast electron and ion transport, and a large number of electroactive sites. The RGO/NiMoS4 composite delivered a specific capacitance of 1273 F g−1 at a current density of 1.0 A g−1, high-rate capability of 71.3% retention from 1 to 6.5 A g−1. Besides, an asymmetric supercapacitor device had a capacitance of 80 F g−1 at a current density of 1.0 A g−1 and an energy density of 82.5 Wh kg−1 at a power density of 1000 W kg−1 with an operating voltage of 1.6 V. Thus, the current RGO/NiMoS4 nanocomposite has a great potential for energy storage application.
Export citation and abstract BibTeX RIS
1. Introduction
Supercapacitors (SCs), also known as electrochemical capacitors, have been widely studied for alternative energy storage devices because they possess high power capabilities, high charge/discharge rates, and very long cycle-life [1–6]. However, SCs still have a low energy density and are not yet practical applications. There are two main strategies to improve the supercapacitors' energy density, including increasing the specific capacitance and enlarging the working window potential. While the capacitance is mainly due to the characteristics of the active electrode materials, the operating window potential is dependent on the properties of electrolytes and the structure of supercapacitors.
In the current study, we proposed to prepare a nanomaterial with high capacitance, high conductivity, and excellent porosity for supercapacitors. The prepared composite consists of bimetallic sulfides (NiMoS4) and reduced graphene sheets (RGO). It was reported that NiMoS4 had an excellent theoretical specific capacitance because of its Faradaic redox reactions with multiple valence states and active sites. Besides, this material is abundant and environmentally friendly [7–9]. However, NiMoS4 shows a low intrinsic electric conductivity [10, 11]. The combination of NiMoS4 and high conducting materials such as graphene is studied to overcome this issue [2, 10].
Xu et al have recently reported preparing NiMoS4/RGO composite by the hydrothermal method [10]. The optimally designed composite showed a high specific capacity of 124 mAh g−1 at 1 A g−1. However, this method required many steps and a long-time reaction that may need a high-cost process. This paper used a fast and effective microwave-assisted one-step approach to prepare high porous RGO/NiMoS4 nanocomposites. Ethylene glycol was used as a green solvent to minimize the use of organic solvents. The prepared nanocomposites provide a high surface area to facilitate the fast electron transport and electrolytes loading in the electrodes. The obtained product is believed to be a promising active material for high-performance supercapacitors.
2. Experimental
2.1. Materials
Nickel (II) nitrate hexahydrate (Ni(NO3).6H2O, 98%, Alfa Aesar), sodium molybdate (Na2MoO4·2H2O, ≥ 98%, Aldrich), thioacetamide (TAA, C2H5NS, 98%, Aldrich) and graphite (> 99%, Alfa Aesar) were used without further purification. The other reagents were of analytical grade. Graphene oxide (GO) was prepared from graphite by an improved Hummers method [12].
2.2. Preparation of NiMoS4/RGO nanocomposites
In a typical experiment, Ni(NO3).6H2O (1.0 mmol), Na2MoO4 (1.0 mmol), TAA (4 mmol), 2.0 ml ethylenediamine, and a varied amount of GO were added into 30 ml ethylene glycol solution and sonicated for 15 min. After that, this mixture was transferred to a 100 ml flask and heated in a microwave at 180 W, 150°C, 15 min. Since the mixture cooled to room temperature, the product was filtered and washed with distilled water and absolute ethanol several times and dried at 65°C for 10 h. Other samples were prepared using 0, 15, 30, and 45 mg of GO, named NG-0, NG-1, NG-2, and NG-3.
2.3. Characterisation
The morphology of prepared materials was examined by scanning electron microscopy (SEM, Hitachi, S-4200), transmission electron microscopy (TEM, JEM 1400, JEOL). The composition and structure of obtained samples were characterized by X-ray photoelectron microscopy (XPS, Kratos AXIS ULTRA), Fourier-transform infrared spectroscopy (FTIR spectrometer, Thermo-Scientific Nicolet IS10), and X-ray diffraction (PANalytical, X'Pert-PRO MPD).
2.4. Electrochemical measurements
The electrochemical measurements, including cyclic voltammetry (CV), galvanostatic charge/discharge (GCD) cycles, and electrochemical impedance spectroscopy (EIS), were performed using a PGSTAT100N (Netherlands) in both three-electrode and two-electrode cells. The electrolyte is 6 M KOH solution. The counter and reference electrodes were a platinum plate and a silver chloride electrode (AgCl/Ag). The working electrodes were prepared by mixing 80 wt% active material with 15 wt% acetylene black (> 99.9%), 5 wt% Nafion solution, and 1 ml ethanol (99.9%) to form a black slurry. The resulting paste was pressed on nickel foam (NF, 1 cm × 1 cm) and dried at 60°C for 10 h. Each electrode contained 1.5 mg of active material. All the NF collectors were cleaned with 3 M HCl solution in an ultrasonic bath for 10 min and washed with DI water, and then dried in a vacuum at 60°C overnight before coating the composites.
The measurements were carried out at room temperature. The specific capacitance (Cs ) was calculated according to the equation: C = It/mΔV, where I, t, m, and ΔV are the applied current (A), discharge time (s), the composite mass (g), and voltage window (V), respectively.
An asymmetric supercapacitor (ASC) was fabricated using the NG-2 as active cathode material and the RGO as active anode material. The cathode and anode electrodes are prepared by the same procedure as described above. Two electrodes were directly packed with the filter paper separator between them.
The energy density (E, Wh kg−1) and power density (P, kW kg−1) are calculated according to the equations:
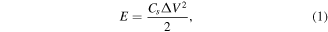
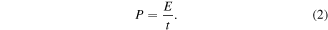
where Cs , ΔV and t are specific capacitance (F g−1), potential range (V), and discharge time (h).
3. Results and discussions
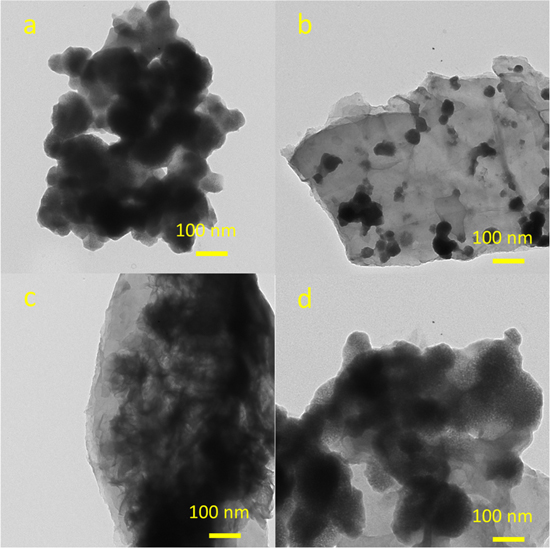
The morphology of the bare NiMoS4 and various RGO/NiMoS4 nanocomposites can be observed by SEM images (figure 1) and more clearly in TEM images (figure 2). The bare NiMo2S4 is in the structure of agglomerated particles (figure 1(a)). After the RGO loadings, the morphology of the prepared nanocomposites was significantly changed. Various forms of the NiMoS4 covered the surface of RGO sheets. At a small amount of RGO loading (figure 1(b)), many NiMoS4 particles were coated on RGO sheets' surface. However, the composite had a hierarchical porous structure with an even distribution of sheet-like NiMoS4 on the surface of RGO sheets (figures 1(c) and (d)) at the GO precursor's extensive contents. The NiMoS4 nanosheets are of several nanometers in thickness and are intercrossed together. More clear structures of the prepared composites are presented in the TEM images, as shown in figure 2. The RGO/NiMoS4 composite's porous structures provide a large open space and many electroactive surface sites, shortening the electrolytes' movement in the electrodes during redox reactions and charge/discharge processes. It will also increase the contact interfaces between electrodes and electrolytes, which will improve electrochemical performance [13]. The NG-2 sample showed the best porous and uniform layer of NiMoS4 nanosheets distributed on the graphene surface. Thus, it was used for other characterizations and tested for electrochemical properties.
Figure 1. SEM images of (a) bare NiMoS4 (NG-0) and the different RGO/NiMoS4 composites: (b) NG-1, (c) NG-2, (d) NG-3.
Download figure:
Standard image High-resolution imageFigure 2. TEM images of (a) bare NiMoS4 (NG-0) and the different RGO/NiMoS4 composites: (b) NG-1, (c) NG-2, (d) NG-3.
Download figure:
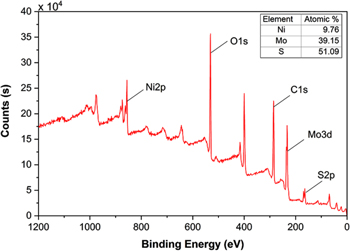
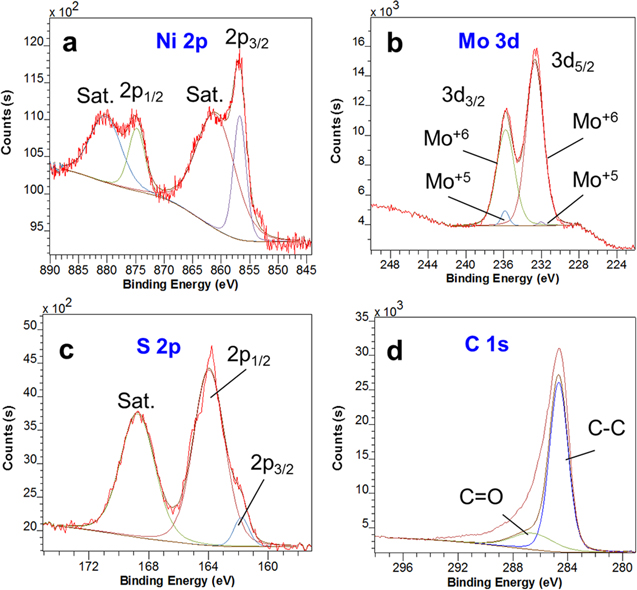
Standard image High-resolution imageThe surface information and the oxidation state of the RGO/NiMoS4 nanocomposite are conducted using XPS, as shown in figures 3 & 4. Figure 3 presented the XPS survey of the RGO/NiMoS4 composite. The main atomic compositions are Ni (9.76%), Mo (39.15%), and S (51.09%). The observed peaks of Ni 2p, Mo 2p, S 2p, C 1 s, and O 1 s confirmed nickel, molybdenum, sulfur, carbon, and oxygen elements existing on the obtained sample's surface. For their oxidation states, the high resolutions of Ni 2p, Mo 3d, S 2p, and C 1 s peaks are displayed in figure 4. The Ni 2p spectrum (figure 4(a)) can be deconvoluted into two peaks at binding energies of 856.8 (2p3/2) and 874.0 eV (2p1/2) according to the Ni2+ spin-orbit doublets. Also, there are two shake-up satellites (labelled as 'Sat.') that appeared at 861.6 and 880.5 eV due to the Ni3+ active species, respectively [13–15]. The Mo 3d spectrum comprised two peaks at binding energies of 232.6 (3d3/2) and 235.6 eV (3d5/2) with a splitting width of approximately 3.0 eV that are attributed to Mo6+ and Mo5+ ions (figure 4(b)) [15, 16]. The high-resolution S 2p peak can be deconvoluted into peaks at 163.5 (2p1/2) and 162.1 eV (2p3/2), indicating the presence of terminal S2− ion. Figure 4(d) shows the C 1 s spectrum with a prominent peak at 284.5 eV (C–C bonds) and a weak peak at 286.7 eV (C=O bonds), which indicates the reduction of GO. The O 1 s peak is according to the multiplicity of adsorbed water at or near the surface. Thus, the XPS analysis confirms the prepared nanocomposite comprised Ni2+, Ni3+, Mo5+, Mo6+, and S2− ions.
Figure 3. XPS survey of the NG-2 nanocomposite.
Download figure:
Standard image High-resolution imageFigure 4. High-resolution XPS spectra of (a) Ni 2p, (b) Mo 3d, (c) S 2p and (d) C 1 s.
Download figure:
Standard image High-resolution imageFigure 5 presents the XRD patterns of GO, bare NiMoS4, and RGO/NiMoS4 (NG-2). The GO spectrum shows a sharp peak at 11.86o (figure 5(a)). The diffraction peaks of bare NiMoS4 were observed at 2θ of 12.78, 33.87, 36.37, and 44.74o that are assigned to MoS2 (JCPDS card no. 24-0513) [17]. Other several diffraction peaks appeared at 2θ of 18.04, 26.45, and 34.80o, according to MoO3 (JCPDS card no. 05-0508) [18]. Besides, some well-defined peaks of Ni2S3 (JCPDS card no. 44-1418) were found at 2θ of 22.15, 28.75, 38.51, 43.15, 49.13, and 55.73o [19]. For the RGO/NiMoS4 composite, the XRD pattern exhibited an excellent assignment with the bare NiMoS4 spectrum's prominent peaks as described above. Besides, the characteristic peak of GO at 2θ of 11.86o disappeared, indicating the reduction of GO. These results suggested the formation of the RGO/NiMoS4 nanocomposite. Also, the chemical structure of samples was investigated by FTIR spectra (Fig. S1). Compared to the spectra of RGO and NiMoS4, the RGO/NiMoS4 has two peaks at 605 and 1035 cm-1 that correspond to the Ni-S or Mo-S vibrations. Besides, the peaks at 1045 (C=O stretching) and 1747 cm−1 (C–O stretching) in the GO structure disappeared, confirming the formation of RGO.
Figure 5. XRD patterns of (a) GO, (b) bare NiMoS4, and (c) the NG-2 composite.
Download figure:
Standard image High-resolution imageThe electrochemical properties were first determined in a three-electrode system. The as-prepared composites were coated as active materials on working electrodes. The Ag/AgCl electrode and platinum foil were used as counter and reference electrodes, respectively. Figure 6(a) shows the CV curves of bare NiMoS4 and different composites at the same scan rate of 5 mV s−1. All electrodes display a typical pseudocapacitive characteristic. At this scan rate, an exact couple of redox peaks is seen. These redox peaks were assigned to the redox conversions of Mo5+/Mo6+ and Ni2+/Ni3+ ion couples. The NG-2 electrode exhibited the highest responding current. It can be explained that conductive RGO sheets coated with the porous and uniform NiMoS4 nanosheets accelerated electron and ion transport, resulting in improved electrochemical performance.
Figure 6. (a) CV curves of the different samples at the same scan rate of 5 mV S−1 in 6 M KOH, (b) Nyquist plots of the various electrodes, (c) the plot of the anodic and cathodic peak currents as a function of the potential scan rates of the NG-2 sample, (d) galvanostatic specific capacitance retention of the NG-2 electrode at different current densities.
Download figure:
Standard image High-resolution imageEIS curves determine the electron resistances of the electrodes and the electrode/electrolyte interface. The high-frequency semicircle is attributed to the charge transfer resistance (Rct), while the low-frequency line corresponds to capacitance behavior. Besides, the intercept of a semicircle with the real axis is an equivalent series resistance (ESR). The ESR includes the electrolyte solution's resistance, the active material's intrinsic resistance, and the active material/current collector's contact resistance. Figure 6(b) presents the EIS plots of NiMoS4 and different RGO/NiMoS4 electrodes measured at a frequency range of 10 kHz to 10 MHz at 5 mV in a 6 M KOH solution. The ESR of all composites is much smaller than that of bare NiMoS4, suggesting lower diffusion resistance and Rct between the electrolyte and the active composites. It can be explained that the RGO nanosheets enhanced the conductivity and porosity of the prepared composite. Moreover, the semicircle diameter in RGO/NiMoS4 curves is smaller than that of bare NiMoS4, indicating the rapid charge transport in the RGO/NiMoS4 structure.
Figure 6(c) presents the anodic/cathodic peak currents versus the potential scan rates. All peak currents are linearly proportional to the scan rates, indicating the composite's rich redox reactions during the charging/storing process. Moreover, there are still typical redox peaks observed at a high scan rate of 100 mV s−1, suggesting a fast electron and ion movement in the electrode structure, good reversibility of redox reactions, and excellent rate capability [20, 21]. The electrochemical performance of the NG-2 electrode is determined by CP measurements at five current densities, as shown in figure 6(d).
The specific capacitance (Cs) could be calculated from the discharge curves. The Cs of 1273, 1003, 970, 933, and 780 F g−1 can achieve at a current density of 1, 1.5, 2.5, 3.5, and 6.5 A g−1, respectively. The rough comparison of the prepared material's electrochemical performance and some published related works is presented in table S1. The Cs value of the RGO/NiMoS4 composite is higher than those of some nickel-molybdenum sulfides. The prepared electrode showed a high capacitance rate with a 61.2% retention from 1.0 to 6.5 A g−1. The enhanced capacitance rate is due to the rapid charge movement in a high porosity and conductivity of the RGO/NiMoS4 composite.
The electrochemical properties were second measured in a two-electrode system. An ASC device was prepared using RGO/NiMoS4 (NG-2) as the positive electrode, RGO as the negative electrode. A 6 M KOH solution was used as the electrolyte. The mass proportion of the positive and negative electrodes was determined to achieve good performance based on the charge balance of q+ = q−. The charge stored by two electrodes (q) can be calculated by the following [19]: q = IΔt = Cs .ΔV.m. Therefore, the mass proportion is calculated by the equation [22]:
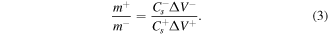
From a three-electrode system, at the current density of 1 A g−1, the Cs values are 1146 F g−1 (NG-2) and 172 F g−1 (RGO). The potential windows are 0–0.5 V (NG-2) and 0–1.0 V (RGO) based on the CV results. Thus, the optimum mass ratio of the cathode and anode electrodes is approximately 0.30. The electrochemical performance of this ASC device was presented in figure 7. From the CV curves at different potential windows (figure 7(a)), the device's full voltage window is 1.5 V. Figure 7(b) presents the CV curves measured at different scan rates, ranging from 2 to 100 mV s−1. Redox peaks can be seen on the CVs even at 100 mV s−1, indicating the pseudocapacitive characteristic attributed to the NG-2 electrode. Besides, the CV curves' shapes were unchanged much even at a high scan rate, confirming an excellent rate capability of the device.
Figure 7. (a) CV curves of the NG-2//AC device at different voltage windows in 6 M KOH, (b) CV curves of the NG-2//AC device at different scan rates in 6 M KOH, (c) galvanostatic discharge curves of the NG-2//AC device at different current densities, (d) average specific capacitance versus the cycle number of the device at a current density of 4 A g−1 (the inset is Nyquist plot of the device before and after 3500 cycles).
Download figure:
Standard image High-resolution imageFigure 7(c) shows the ASC device's CP profiles at various current densities from 1 to 4 A g−1. The Cs is 80 F g−1 at 1 A g−1 and maintains 80.6% (325 F g−1) at 4 A g−1. The ASC device's cycling performance was tested at the current density of 4 A g−1 (figure 7d). The capacitance remains over 62% after 3500 cycles, indicating the ASC's good stability. On the other hand, the device's EIS curves before and after 3500 cycles have a similar shape, suggesting the obtained device's good cyclic stability. The device exhibits an E of 82.5 W h kg−1 at a P of 1000 W kg−1, which is better than those of previous related reports (as shown in Fig. S2), such as Co3S4/CoMo2S4-rGO//AC (33.1 W h kg−1 at 850 W kg−1) [22], CoMoS4//AC (27.2 W h kg−1 at 400 kW kg−1) [23], Co9S8/NSA//AC (20.0 W h kg−1 at 828.5 W kg−1) [24], and Co-Mo-S//AC (42.6 W h kg−1 at 850.3 kW kg−1) [25], suggesting a great potential of our ASC device for practical supercapacitor applications.
4. Conclusions
A high mesoporous RGO/NiMoS4 nanomaterial was prepared successfully by an effective microwave-assisted approach. The prepared composite showed a high specific capacitance of 955 F g−1 at 1.0 A g−1 in a three-electrode measurement. The ASC device packed using RGO/NiMoS4 cathode and RGO anode has an energy density of 82.5 Wh kg−1 at a power density of 1000 W kg−1. Besides, the device showed good cyclic stability of 62% after 3500 cycles. The prepared composite appears as a high potential nanomaterial for supercapacitor application.
Acknowledgments
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.99-2018.310.